Autograft-matching wireless bioelectronic conduits: Magneto-electric coupling enabled taurine metabolism activation for peripheral nerve repair
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Peripheral nerve injuries remain a clinical challenge due to the limitations of autografts and unstable electrical signals in existing bioelectronic therapies. It is necessary to develop innovative strategies to achieve wireless, controllable peripheral neural regeneration (PNR). In this study, we developed a magneto-electric coupling-driven electroactive nerve guidance conduits (PCLG/AgNF NGCs) with a moving magnetic field (MMF) for PNR with wireless magneto-electric coupling electrical stimulation (MECES). PCLG/AgNF displayed high conductivity (25.48 ± 3.77 S/cm) and wireless controllability of generating electrical pulses (16.67 ± 0.47 μA to 475.7 ± 9.71 μA) with an MMF. The MECES produced by PCLG/AgNF with the MMF significantly promoted cell proliferation, cell migration, and upregulated the expression of β3-tubulin, neurofilament heavy chain and growth-associated protein 43, compared to PCLG/AgNF and MMF used individually. Mechanistically, we identified that PCLG/AgNF with the MMF activated the metabolism of taurine and hypotaurine corroborated by elevated intracellular taurine, which is crucial for MECES mediated repair processes. In a rat peripheral nerve defect model, the PCLG/AgNF NGCs with the MMF showed promising results in nerve regrowth, myelination, and functional recovery, performing comparably to autografts. This strategy offers PCLG/AgNF NGCs as a wireless, controllable, precision-enabled approach and provides novel insights for the effective PNR.
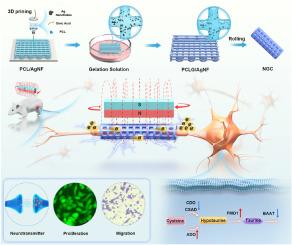
自体移植物匹配无线生物电子导管:磁电耦合激活牛磺酸代谢修复周围神经
由于自体移植物的局限性和现有生物电子疗法中电信号的不稳定,周围神经损伤仍然是一个临床挑战。为了实现无线、可控的周围神经再生(PNR),有必要开发创新的策略。在这项研究中,我们开发了一种具有移动磁场(MMF)的磁电耦合驱动电活动神经引导导管(PCLG/AgNF NGCs),用于无线磁电耦合电刺激(MECES)的PNR。PCLG/AgNF具有高电导率(25.48±3.77 S/cm)和无线可控性(16.67±0.47 μA ~ 475.7±9.71 μA)。与单独使用PCLG/AgNF和MMF相比,PCLG/AgNF与MMF联合产生的MECES可显著促进细胞增殖、细胞迁移,上调β3-微管蛋白、神经丝重链和生长相关蛋白43的表达。在机制上,我们发现PCLG/AgNF与MMF一起激活牛磺酸和低牛磺酸的代谢,细胞内牛磺酸升高证实了这一点,这对MECES介导的修复过程至关重要。在大鼠周围神经缺损模型中,PCLG/AgNF NGCs与MMF在神经再生、髓鞘形成和功能恢复方面表现出与自体移植物相当的良好效果。该策略为PCLG/AgNF NGCs提供了一种无线、可控、精确的方法,并为有效的PNR提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


