Synthesis, DFT and molecular docking studies of N-Heterocyclic carbene selenium compounds conferring anticancer and antibacterial activity
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Target based drug design is an important strategy that increases the selectivity, efficacy and safety of drug candidates. In this study we designed synthesis of benz-imidazolium salts (L1-L3) and selenium compounds (C1–C3) to investigate their enzyme inhibition potential and bioactivity. Successful synthesis was confirmed through analytical techniques like UV–Vis., FTIR, 1H & 13C NMR and mass spectrometry that further supported by computational (DFT) studies. Molecular docking studies of C1–C3 against key molecular targets (COX-1, EGF, VEGF and HIF) was conducted. Among the test compounds C1 showed an impressive binding affinity of −6.14 kcal mol−1 against EGF which is comparable to standard drug 5-FU (−4.97 kcal mol−1). The validation of docking results through in vitro studies confirmed C1 as most potent inhibitor among the test compounds, having inhibition of 67.4 ± 1.3 % and 86.7 ± 1.8 % against COX-1 and EGF respectively. Furthermore, test compounds showed significant inhibition potential against thioredoxin reductase (TrxR). Cytotoxicity profiling across HepG2, HeLa and A-2780 cell lines confirmed C1 as the lead compound, with IC50 values of 0.956, 1.986, and 0.862 μg/mL, respectively. Test compounds also showed antibacterial activity by showing inhibition zone 8.5 ± 1.1–27.0 ± 1.2 mm against E. coli and S. aureus. These findings showed that NHC based selenium compounds could be a potential drug candidate for chemotherapy against multiple cancerous strains.
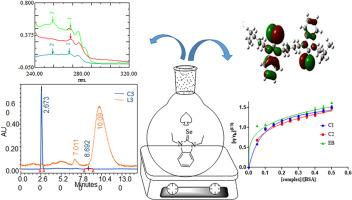
具有抗癌和抗菌活性的n -杂环碳硒化合物的合成、DFT及分子对接研究
基于靶标的药物设计是提高候选药物选择性、有效性和安全性的重要策略。本研究设计了苯-咪唑盐(L1-L3)和硒化合物(C1-C3)的合成,考察了它们的酶抑制潜力和生物活性。通过紫外-可见等分析技术证实合成成功。计算(DFT)研究进一步支持了FTIR、1H & 13C NMR和质谱分析。进行C1-C3与关键分子靶点(COX-1、EGF、VEGF、HIF)的分子对接研究。在测试化合物中,C1对EGF的结合亲和力为- 6.14 kcal mol−1,与标准药物5-FU (- 4.97 kcal mol−1)相当。体外对接结果验证证实C1是被试化合物中最有效的抑制剂,对COX-1和EGF的抑制率分别为67.4±1.3%和86.7±1.8%。此外,测试化合物对硫氧还蛋白还原酶(TrxR)具有显著的抑制潜力。对HepG2、HeLa和A-2780细胞株的细胞毒性分析证实C1为先导化合物,IC50值分别为0.956、1.986和0.862 μg/mL。实验化合物对大肠杆菌和金黄色葡萄球菌的抑制范围为8.5±1.1 ~ 27.0±1.2 mm。这些发现表明,基于NHC的硒化合物可能是一种潜在的候选药物,用于化疗多种癌症菌株。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


