Theoretical study of Ni-modified B12N12 nanocages: Insights into CO capture potential
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The adsorption behavior of carbon monoxide (CO) on pristine and nickel (Ni)-modified B12N12 nanocages was investigated using density functional theory (DFT) calculations at the B97-3c/6-31G(d,p) level. Weak physisorption of CO was observed on pristine B12N12, NiB11N12, and Ni@B12N12 nanocages, whereas strong chemisorption occurred on the B12N11Ni, Ni@b66, and Ni@b64 nanocages. Notably, the Ni@b64 nanocage exhibited the highest adsorption energy and the greatest electronic sensitivity. This system demonstrated the ability to adsorb up to nine CO molecules with average adsorption energies ranging from −1.87 to −0.49 eV per CO molecule, resulting in a maximum CO capture capacity of 43.61 wt% (15.57 mmol/g or 436.10 mg/g). These findings were confirmed by molecular dynamics tests and highlight the excellent potential of Ni-decorated B12N12 nanocages, particularly Ni@b64, as a high-performance material for CO capture, suggesting promising applications in gas separation, storage technologies, and environmental remediation.
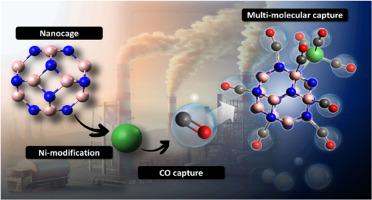
ni修饰B12N12纳米笼的理论研究:对CO捕获潜力的见解
采用密度泛函理论(DFT)在B97-3c/6-31G(d,p)水平上研究了镍修饰B12N12纳米笼对一氧化碳(CO)的吸附行为。在原始的B12N12、NiB11N12和Ni@B12N12纳米笼上观察到CO的弱物理吸附,而在B12N11Ni、Ni@b66和Ni@b64纳米笼上观察到CO的强化学吸附。值得注意的是,Ni@b64纳米笼具有最高的吸附能和最大的电子灵敏度。该系统能够吸附多达9个CO分子,平均吸附能范围为- 1.87至- 0.49 eV / CO分子,最大CO捕获容量为43.61 wt% (15.57 mmol/g或436.10 mg/g)。这些发现得到了分子动力学测试的证实,并突出了ni修饰的B12N12纳米笼(特别是Ni@b64)作为CO捕获的高性能材料的卓越潜力,在气体分离、储存技术和环境修复方面具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


