pH-dependent formation potential of OH∗ on Pt(111): Double layer effect on water dissociation
IF 17.9
2区 材料科学
Q1 Engineering
引用次数: 0
Abstract
The adsorption/desorption of OH∗ on electrode surfaces is pivotal in numerous electrocatalytic reactions. To understand the effect of electrolyte pH on that process, in this work, an advanced approach combining ab initio molecular dynamics (AIMD) with free energy perturbation is employed to calculate the dehydrogenation free energy of water chemisorbed at differently electrified Pt(111)/electrolyte interfaces. Our findings reveal that the onset potential for OH∗ formation shifts negatively as the pH increases at low pH condition (pH4.3), aligning with the cyclic voltammetry curves observed in experimental studies. It indicates the dissociation of chemisorbed water is the primary route for OH∗ adsorption at low pH condition. Furthermore, it is also found that the variation in dehydrogenation energy across different pH is primarily due to the local hydrogen bonding network surrounding the chemisorbed water. In addition, it is proposed that at high pH conditions OH− oxidation emerges as the primary route for OH∗ adsorption on Pt(111) constrained by the water chemisorption process. This work provides crucial insights into the pH-dependent adsorption behavior of OH∗ on the Pt(111) surface and aims to guide the optimization of electrolytes to boost the efficiency of related reactions.
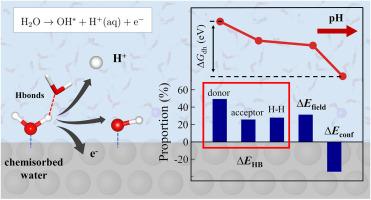
Pt(111)上OH *的ph依赖性形成电位:水解离的双层效应
OH *在电极表面的吸附/解吸是许多电催化反应的关键。为了了解电解质pH值对这一过程的影响,本研究采用了一种结合从头算分子动力学(AIMD)和自由能摄动的先进方法来计算在不同通电Pt(111)/电解质界面化学吸附的水的脱氢自由能。我们的研究结果表明,在低pH条件下(pH<4.3), OH *形成的起始电位随着pH的增加而负移动,这与实验研究中观察到的循环伏安曲线一致。表明在低pH条件下,化学吸附水的解离是OH *吸附的主要途径。此外,还发现脱氢能在不同pH值下的变化主要是由于化学吸附水周围的局部氢键网络。此外,还提出在高pH条件下,OH−氧化成为OH *吸附在Pt(111)上的主要途径,受水化学吸附过程的限制。这项工作为Pt(111)表面OH *的ph依赖吸附行为提供了重要的见解,并旨在指导电解质的优化以提高相关反应的效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Materials Science
Engineering-Mechanics of Materials
CiteScore
20.90
自引率
3.00%
发文量
294
审稿时长
9 weeks
期刊介绍:
Nano Materials Science (NMS) is an international and interdisciplinary, open access, scholarly journal. NMS publishes peer-reviewed original articles and reviews on nanoscale material science and nanometer devices, with topics encompassing preparation and processing; high-throughput characterization; material performance evaluation and application of material characteristics such as the microstructure and properties of one-dimensional, two-dimensional, and three-dimensional nanostructured and nanofunctional materials; design, preparation, and processing techniques; and performance evaluation technology and nanometer device applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


