Modification of copper-based zeolite 13X for methanol and aromatic compound synthesis in microwave plasma–assisted carbon dioxide conversion
Q1 Environmental Science
Case Studies in Chemical and Environmental Engineering
Pub Date : 2025-08-20
DOI:10.1016/j.cscee.2025.101277
引用次数: 0
Abstract
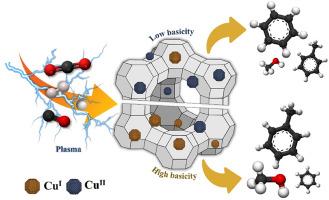
The direct conversion of carbon dioxide (CO2) into valuable products holds significant potential for advancing a sustainable circular carbon economy. Herein, bifunctional catalysts—copper (Cu)-based zeolite 13X—were modified to enhance methanol and aromatic synthesis in microwave plasma–assisted CO2 conversion. The Cu oxidation state and surface basicity of the modified catalysts were adjusted through thiourea reduction and confirmed via X-ray photoelectron spectroscopy, H2 temperature-programmed reduction, and CO2 temperature-programmed desorption techniques. The results of the investigated catalytic activity of modified CuI/II-based zeolite 13X, pristine zeolite 13X, and plasma alone in microwave plasma–catalytic CO2 conversion revealed that the microwave plasma–assisted catalyst could effectively and directly convert CO2 into valuable products—methanol, benzene, and toluene—without intermediate purification. This catalyst system considerably improved CO2 conversion rate (>50 %) compared with only 18.5 % conversion with the plasma alone catalyst, while the %Selectivity toward methanol and aromatics was >45 %. Additionally, the effects of CuI/CuII ratios and basicity concentrations on methanol and aromatic %Selectivity were investigated. The synergy between the CuI species and surface basicity played a crucial role in promoting toluene and methanol production with low benzene production. The comparable catalytic activities of 0.5 wt% Cu doping with thiourea reduction and 6 wt% Cu doping without thiourea reduction highlighted the efficiency of the thiourea reduction process in economically producing catalysts with fewer metal precursors. Finally, the potential pathways for methanol and aromatic formation were proposed, and the feasibility of industrial scale up was discussed.
改性铜基沸石13X用于微波等离子体辅助二氧化碳转化合成甲醇和芳香族化合物
将二氧化碳直接转化为有价值的产品,对于推进可持续的循环碳经济具有巨大的潜力。本文对铜基沸石13x双功能催化剂进行了改性,以促进微波等离子体辅助CO2转化中甲醇和芳香族的合成。通过硫脲还原调整催化剂的Cu氧化态和表面碱度,并通过x射线光电子能谱、H2程序升温还原和CO2程序升温解吸技术进行了验证。对改性CuI/ ii基沸石13X、原始沸石13X和等离子体在微波等离子体催化CO2转化中的催化活性进行了研究,结果表明,微波等离子体辅助催化剂可以有效、直接地将CO2转化为有价产物甲醇、苯和甲苯,而无需中间提纯。与等离子体催化剂的18.5%的转化率相比,该催化剂体系显著提高了CO2的转化率(50%),对甲醇和芳烃的选择性为45%。此外,还考察了崔/崔比和碱度浓度对甲醇和芳香%选择性的影响。崔种与表面碱度之间的协同作用在低苯产量下促进甲苯和甲醇的生产中起着至关重要的作用。0.5 wt% Cu掺杂硫脲还原和6 wt% Cu掺杂不掺杂硫脲还原的催化活性相当,突出了硫脲还原工艺在较少金属前体的情况下经济生产催化剂的效率。最后,提出了合成甲醇和芳烃的可能途径,并讨论了工业规模化的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Case Studies in Chemical and Environmental Engineering
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
103
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


