Enhanced reactivity and electron efficiency of nanoscale zero-valent iron for nitroaromatic compounds reduction through modification with anthraquinone-2-carboxylic acid
IF 7.1
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
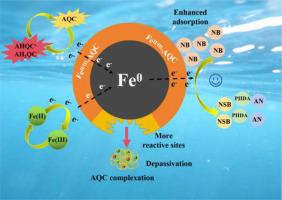
Humic substances (HS) modification favors the reactivity of nanoscale zero-valent iron (nZVI) for water decontamination from nitro-compounds like nitrobenzene (NB), whereas the structural complexity of HS hinders the elucidation of its mechanism. Herein, anthraquinone-2-carboxylic acid (AQC) was employed as a typical model molecule for HS. Through AQC modification, the water contact angle of nZVI evidently increased from 6° to 19°-46°, and the rate constant and electron efficiency for NB reduction under aerobic condition was substantially elevated by 6–10.3 times and 2.5–2.8 times, respectively. Among the AQC-modified nZVI materials with varied molar ratios of AQC/Fe (0.1–3 %), 1 % AQC-nZVI not only exhibited the optimal reactivity and electron efficiency, but also demonstrated enhanced reactivity for various nitroaromatic compounds. Moreover, AQC modification could advance the complete reduction of NB by nZVI to aniline and minimize the accumulation of intermediates. In addition, AQC-nZVI materials could maintain an excellent reduction efficiency over a wide pH range (4–9). Mechanistically, the increase of surface hydrophobicity of nZVI owing to AQC modification promoted the adsorption affinity for NB and redirected more electron transfer to NB. Leveraging both anthraquinone and carboxylic groups, AQC served as electron shuttle to mediate the electron transfer from nZVI to NB whilst accelerating the Fe(II)/Fe(III) circulation. Electrochemical impedance spectroscopy revealed a pronounced decrease in the charge transfer resistance of nZVI from 381.4 Ω to 252.8–337.4 Ω due to AQC modification, thereby facilitating the interfacial electron transfer. This study offers insights into the HS-enhanced reactivity and selectivity of nZVI for water decontamination from nitroaromatic compounds.
蒽醌-2-羧酸修饰纳米级零价铁还原硝基芳香族化合物的反应活性和电子效率提高
腐殖质(HS)改性有利于纳米级零价铁(nZVI)对硝基苯(NB)等硝基苯类化合物的水净化,但HS结构的复杂性阻碍了其机理的阐明。本文以蒽醌-2-羧酸(AQC)作为HS的典型模型分子。通过AQC改性,nZVI的水接触角从6°明显增加到19°~ 46°,好氧条件下NB还原速率常数和电子效率分别大幅提高了6 ~ 10.3倍和2.5 ~ 2.8倍。在不同AQC/Fe摩尔比(0.1 ~ 3%)的AQC-nZVI材料中,1% AQC-nZVI不仅表现出最佳的反应活性和电子效率,而且对各种硝基芳香族化合物的反应活性也有所提高。此外,AQC改性可以促进NB被nZVI完全还原为苯胺,并减少中间体的积累。此外,AQC-nZVI材料在较宽的pH范围内(4-9)都能保持优异的还原效率。机理上,AQC修饰提高了nZVI的表面疏水性,促进了对NB的吸附亲和力,并将更多的电子转移到NB上。利用蒽醌和羧基,AQC作为电子穿梭器,介导电子从nZVI到NB的转移,同时加速Fe(II)/Fe(III)循环。电化学阻抗谱显示,由于AQC修饰,nZVI的电荷转移电阻从381.4 Ω明显降低到252.8-337.4 Ω,从而促进了界面电子的转移。本研究揭示了hs增强的nZVI对硝基芳香族化合物水中净化的反应性和选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


