MicroRNA-93-5p alleviates uric acid-induced fibrosis in renal tubular epithelial cells by regulating the SMAD5/Id2 signaling pathway
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Hyperuricemia (HUA) is a prevalent metabolic disorder that contributes significantly to renal injury and may lead to the development of chronic kidney disease. Although previous studies have explored this condition, the molecular mechanisms underlying HUA-induced renal damage, particularly the role of small RNAs, remain inadequately understood. This study aimed to investigate the potential role of microRNA-93-5p (miR-93-5p) in HUA-associated renal injury. A comprehensive analysis was conducted to examine changes in miR-93-5p expression and its target gene, SMAD5, using knockout mouse models, cell cultures, transgenic techniques, and molecular biology methods. The findings demonstrated a significant upregulation of miR-93-5p in both HUA models and clinical serum samples, with a strong correlation to renal injury. Deletion of miR-93-5p resulted in increased SMAD5 expression and improved renal function, indicating that miR-93-5p contributed to renal injury by suppressing SMAD5 and promoting partial epithelial-mesenchymal transition in renal tubular epithelial cells. Transcriptomic analysis further revealed that miR-93-5p regulated numerous genes associated with inflammation, fibrosis, and apoptosis. Overall, these results underscore the pivotal role of miR-93-5p in HUA-induced renal damage through modulation of the SMAD5/Id2 signaling pathway, offering valuable insights into kidney disease pathophysiology and identifying potential therapeutic targets for clinical intervention.
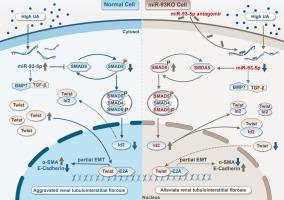
MicroRNA-93-5p通过调节SMAD5/Id2信号通路减轻尿酸诱导的肾小管上皮细胞纤维化
高尿酸血症(HUA)是一种普遍存在的代谢性疾病,对肾脏损伤有重要作用,并可能导致慢性肾脏疾病的发展。尽管先前的研究已经探索了这种情况,但hua诱导肾损害的分子机制,特别是小rna的作用,仍然没有得到充分的了解。本研究旨在探讨microRNA-93-5p (miR-93-5p)在hua相关性肾损伤中的潜在作用。采用敲除小鼠模型、细胞培养、转基因技术和分子生物学方法,对miR-93-5p及其靶基因SMAD5的表达变化进行了全面分析。研究结果表明,在HUA模型和临床血清样本中,miR-93-5p均显著上调,且与肾损伤有很强的相关性。miR-93-5p的缺失导致SMAD5表达增加和肾功能改善,表明miR-93-5p通过抑制SMAD5和促进肾小管上皮细胞部分上皮-间质转化参与肾损伤。转录组学分析进一步显示,miR-93-5p调节了许多与炎症、纤维化和细胞凋亡相关的基因。总的来说,这些结果强调了miR-93-5p通过调节SMAD5/Id2信号通路在hua诱导的肾损伤中的关键作用,为肾脏疾病病理生理学提供了有价值的见解,并为临床干预确定了潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


