Spontaneous dynamic modulation of ionic/immune microenvironment on polyetheretherketone for sequential anti-infection and osseointegration
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Initial implant-related infection and subsequent poor osseointegration are the main causes of implant placement failure. The extracellular microenvironment is an important mediator of behaviors of cell and bacteria; however, spontaneously dynamically regulating the microenvironment to match tissue integration processes remains a challenge. Here, we construct a multilayer film on polyetheretherketone (PEEK) surface with different inner and outer layers of magnesium oxide (MgO) degradation rates. This film can sequentially regulate surface ions and immune microenvironment to achieve sequential antibacteria and bone integration. In the early stage of bone implantation, the outer layer of MgO can rapidly degrade to produce a strong alkaline microenvironment and a large amount of magnesium (Mg) ions, disrupting the energy metabolism of adherent bacteria and inducing M1 polarization of macrophages to enhance their ability to engulf planktonic bacteria. In the later stage, the inner layer MgO can slowly release Mg ion for a long time, synergistically promoting the proliferation and differentiation of osteoblasts by directly stimulating osteoblasts and inducing M2 polarization of macrophages. The rat femoral implantation model confirms the good sequential immune-enhanced antibacteria and bone integration ability of the film in vivo. In addition, the film can control the polarization time of cells by adjusting the thickness of the outer layer to meet the needs of different scenarios. This study demonstrates that the synergistic induction of ion microenvironment and immune microenvironment is a promising and safe surface modification strategy for bone implants.
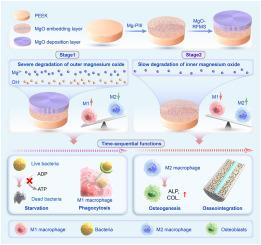
离子/免疫微环境对聚醚醚酮序列抗感染和骨整合的自发动态调节
最初的种植体相关感染和随后的骨整合不良是种植体植入失败的主要原因。细胞外微环境是细胞和细菌行为的重要中介;然而,自发地动态调节微环境以匹配组织整合过程仍然是一个挑战。本文在聚醚醚酮(PEEK)表面构建了具有不同内层和外层氧化镁(MgO)降解率的多层膜。该膜可顺序调节表面离子和免疫微环境,实现顺序抗菌和骨整合。在植骨早期,MgO外层可以快速降解,产生强碱性微环境和大量镁离子,破坏贴壁细菌的能量代谢,诱导巨噬细胞M1极化,增强其吞噬浮游细菌的能力。在后期,内层MgO可以长期缓慢释放Mg离子,通过直接刺激成骨细胞和诱导巨噬细胞M2极化,协同促进成骨细胞的增殖和分化。大鼠股骨植入模型证实了该膜在体内具有良好的序贯免疫增强抗菌和骨整合能力。此外,该薄膜还可以通过调节外层厚度来控制电池的极化时间,以满足不同场景的需要。本研究表明,离子微环境和免疫微环境的协同诱导是一种有前途的、安全的骨种植体表面修饰策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


