Indigenous African chickens (Gallus gallus domesticus) lack an anti-Trypanosoma factor and have a prospect for zoonotic transmission of African trypanosomiasis
IF 1.6
4区 医学
Q3 PARASITOLOGY
引用次数: 0
Abstract
African trypanosomes evade host immune response through antigenic variation and utilize other immune modulatory mechanisms to survive in the immunologically hostile mammalian bloodstream. However, indigenous African chicken (Gallus gallus domesticus) exhibits lower susceptibility to trypanosomes, suggesting unique resistance mechanisms; but the exact factor(s) of resistance remains elusive. This study aims to elucidate the mechanisms underlying the observed resistance of indigenous African chickens to T. brucei brucei infection, and to assess their potential role as cryptic reservoirs in zoonotic transmission. Non-immunosuppressed and immunosuppressed chickens were intravenously inoculated with ∼2.5 × 108 parasites, and parasitemia was monitored using microscopy, xenodiagnosis, and PCR. Rats served as controls and were intraperitoneally infected with 104 parasites. Haematological parameters in both chickens and rats were assessed using standard methods. Furthermore, in vitro anti-Trypanosoma activity of normal and infected chicken blood components was evaluated. The results reveal that chickens displayed no microscopic parasitemia beyond 9 h post-infection (pi) and survived beyond 60 days, whereas rats passaged with over 104-folds less trypanosomes developed parasitemia at day 5, which progressed and killed the rats between days 10–19. Further, while there were no significant haematological alterations over a 4-week observation period in the chicken, infected rats presented significant reductions in packed cell volume, haemoglobin, and red blood cell counts at peak infection, indicating anaemia sequelae. Additionally, infected rats exhibited neutropenia, lymphocytosis, increased hemolysis and mortality. Intriguingly, despite the observed trypanosomes suppression in chickens, incubation of trypanosomes with chicken blood, serum, or plasma revealed no intrinsic anti-Trypanosoma activity. But blood collected from infected chickens at 1- and 7-days post-infection successfully initiated infection in rats through xenodiagnosis, confirming transmissibility despite the absence of detectable parasitemia in chickens revealing a covert but potentially infectious state. Similarly, PCR detection at 7 dpi, indicated covert/suppressed infection. These findings suggest that indigenous African chickens, while resistant to overt trypanosomiasis, may act as cryptic reservoirs for Trypanosoma spp., potentially facilitating parasite zoonotic transmission.
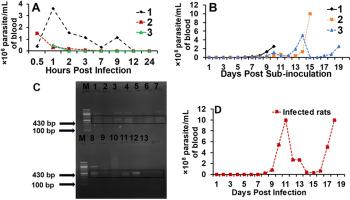
非洲本土鸡(Gallus Gallus domesticus)缺乏抗锥虫因子,具有非洲锥虫病人畜共患传播的前景
非洲锥虫通过抗原变异逃避宿主免疫反应,并利用其他免疫调节机制在免疫敌对的哺乳动物血液中存活。然而,非洲本土鸡(Gallus Gallus domesticus)对锥虫的易感性较低,表明其独特的抗性机制;但是产生抗药性的确切因素仍然难以捉摸。本研究旨在阐明已观察到的非洲土生鸡对布氏体感染产生耐药性的机制,并评估其作为人畜共患病传播的隐宿主的潜在作用。将非免疫抑制鸡和免疫抑制鸡静脉接种约2.5 × 108种寄生虫,利用显微镜、异种诊断和PCR监测寄生虫血症。以大鼠为对照,腹腔内感染104种寄生虫。采用标准方法测定鸡和大鼠的血液学参数。此外,还比较了正常和感染鸡血液成分的体外抗锥虫活性。结果表明,鸡在感染后9小时内未出现微观寄生虫病,存活时间超过60天,而大鼠在感染后第5天出现寄生虫病,并在10-19天之间进展并死亡。此外,虽然在4周的观察期内,鸡的血液学没有明显改变,但感染大鼠在感染高峰时,堆积细胞体积、血红蛋白和红细胞计数明显减少,表明有贫血后遗症。此外,感染大鼠表现出中性粒细胞减少、淋巴细胞增多、溶血增加和死亡率。有趣的是,尽管在鸡体内观察到锥虫的抑制作用,但在鸡的血液、血清或血浆中孵育锥虫并没有显示出内在的抗锥虫活性。但是,在感染后1天和7天从感染鸡身上采集的血液通过异种诊断成功地启动了大鼠的感染,证实了传染性,尽管在鸡身上没有可检测到的寄生虫血症,揭示了一种隐蔽但潜在的感染状态。同样,PCR检测在7 dpi,表明隐蔽/抑制感染。这些发现表明,非洲本土鸡虽然对明显的锥虫病有抵抗力,但可能是锥虫的隐宿主,可能促进寄生虫人畜共患传播。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental parasitology
医学-寄生虫学
CiteScore
3.10
自引率
4.80%
发文量
160
审稿时长
3 months
期刊介绍:
Experimental Parasitology emphasizes modern approaches to parasitology, including molecular biology and immunology. The journal features original research papers on the physiological, metabolic, immunologic, biochemical, nutritional, and chemotherapeutic aspects of parasites and host-parasite relationships.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


