Multifunctional, enzyme/pH-responsive gelatin microspheres with aptamer-targeted antibacterial and ionic-mediated dual therapy for infected bone defects
IF 12.9
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The treatment of infectious bone defects requires simultaneous resolution of bacteria-associated antibiotic resistance, inflammatory microenvironment dysregulation, and impaired bone regeneration. Here, we developed an injectable, self-assembling designed gelatin micro-/nano-sphere system (GHMs@G1-N-A/T) that addresses the tripartite challenges of infectious bone defects through innovative material engineering: Antibacterial module featuring aptamer-conjugated gelatin nanospheres (AGN-Apt/Te) for MRSA-specific targeting, coupled with dual enzyme/pH-responsive release mechanisms (gelatinase-triggered nanosphere detachment and MgO2-derived ROS generation); A self-assembling microsphere scaffold (GHMs) constructed through vanillin-mediated crosslinking and nano-hydroxyapatite (n-HA)/MgO2 incorporation, enabling sequential release of Mg2+/Ca2+; and A gelatinase-sensitive peptide (G-1) interface that dynamically links these components, ensuring microenvironment-responsive functionality. Results demonstrated that gelatinase-triggered AGN-Apt/Te nanospheres detachment enabled bacteria-specific antibiotic delivery, achieving greater than 95 % eradication of S. aureus and MRSA through synergistic biofilm disruption (via MgO2-derived ROS bursts) and Te-mediated membrane damage. In vitro, self-assembling GHMs scaffold ensured sustained release of Mg2+/Ca2+, thereby promoting HUVEC tube formation (1.9-fold) and osteogenic differentiation of BMSCs. In a rat osteomyelitis model, GHMs@G1-N-A/T demonstrated sequential therapeutic efficacy: rapid infection control (greater than 95 % reduction within 7 days) followed by functional bone regeneration (46.32 % BV/TV at day 28). This work offers a new multifunctional biomaterial design that integrates hierarchical modular assembly, infection microenvironment-responsive logic and sequential transition from antibacterial to regenerative for the repair of complex infectious bone defects.
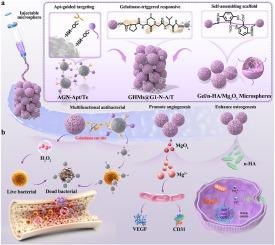
多功能,酶/ ph响应明胶微球与适体靶向抗菌和离子介导双重治疗感染骨缺损
感染性骨缺损的治疗需要同时解决细菌相关的抗生素耐药性、炎症微环境失调和骨再生受损。在这里,我们开发了一种可注射的,自组装设计的明胶微/纳米球系统(GHMs@G1-N-A/T),通过创新的材料工程解决感染性骨缺陷的三方面挑战:抗菌模块具有适配体共轭明胶纳米球(AGN-Apt/Te),用于mrsa特异性靶向,结合双酶/ ph响应释放机制(明胶酶触发的纳米球脱离和mgo2衍生的ROS生成);通过香草素介导的交联和纳米羟基磷灰石(n-HA)/MgO2结合构建自组装微球支架(GHMs),实现Mg2+/Ca2+的顺序释放;明胶酶敏感肽(G-1)接口,动态连接这些组件,确保微环境响应功能。结果表明,明胶酶触发的AGN-Apt/Te纳米球分离能够实现细菌特异性抗生素的递送,通过协同生物膜破坏(通过mgo2衍生的ROS爆发)和Te介导的膜损伤,实现95%以上的金黄色葡萄球菌和MRSA的根除。体外自组装GHMs支架保证了Mg2+/Ca2+的持续释放,从而促进HUVEC管的形成(1.9倍)和BMSCs的成骨分化。在大鼠骨髓炎模型中,GHMs@G1-N-A/T显示出顺序治疗效果:快速感染控制(7天内减少95%以上),然后是功能性骨再生(第28天BV/TV为46.32%)。这项工作提供了一种新的多功能生物材料设计,集成了分层模块化组装,感染微环境响应逻辑和从抗菌到再生的顺序过渡,用于修复复杂的感染性骨缺损。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


