Direct reduction of copper slag with H2 and CO: Effects of CaCO₃ on reduction kinetics
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-08-23
DOI:10.1016/j.jtice.2025.106371
引用次数: 0
Abstract
Background
The choice of reductants and additives as well as the reduction process is critical to reduce energy consumption, improve quality and reduce costs. As the production capacity of copper slag has surged in modern industry, the efficient utilization of iron resources contained within it has emerged as a prominent research focus.
Methods
In this study, copper slag was used as the object and carbon monoxide and hydrogen as the reducing agents, combined with kinetic mathematical modeling, focusing on the advantages of hydrogen reduction of copper slag and the improvement of reduction kinetic conditions by CaCO3.
Significant Findings
The results of the study show that hydrogen reduction is more efficient when copper slag briquettes are directly reduced with H2 and CO; Temperature and CaCO3 had a significant effect on the reduction rate and degree of reduction of copper slag briquettes; In addition, the phase kinetic analysis of copper slag briquettes reveals that the apparent activation energy change rule is Ephase 1 < Ephase 2 > Ephase 3 when CO reduces copper slag; And when H2 reduces the copper slag, the apparent activation energy change rule is Ephase 1 < Ephase 2 < Ephase 3. This indicates that as the reaction proceeds, the product layer inside the copper slag becomes dense, and the product layer is even denser due to the higher reduction of the endpoint in the H2 atmosphere, resulting in an increase in the internal diffusion of the gas and a continuous increase in the apparent activation energy. The apparent activation energy of gas-reduced copper slag increased significantly with the addition of CaCO3.
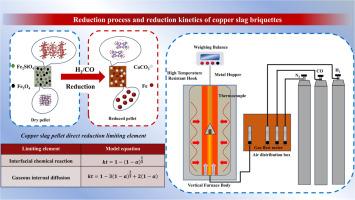
H2和CO直接还原铜渣:CaCO₃对还原动力学的影响
还原剂和添加剂的选择以及还原过程对降低能耗、提高质量和降低成本至关重要。随着现代工业中铜渣生产能力的飞速发展,对铜渣中含铁资源的高效利用已成为一个突出的研究热点。方法本研究以铜渣为研究对象,以一氧化碳和氢气为还原剂,结合动力学数学建模,重点研究氢还原铜渣的优势以及CaCO3对还原动力学条件的改善。研究结果表明:用H2和CO直接还原铜渣型煤时,氢还原效率更高;温度和CaCO3对铜渣型煤的还原速率和还原程度有显著影响;此外,对铜渣型煤的相动力学分析表明,CO还原铜渣时,表观活化能变化规律为Ephase 1 <; Ephase 2 > Ephase 3;H2还原铜渣时,表观活化能变化规律为Ephase 1 <; Ephase 2 < Ephase 3。这说明随着反应的进行,铜渣内部的生成物层变得致密,并且由于H2气氛中端点的还原程度更高,使得生成物层更加致密,导致气体内部扩散增加,表观活化能不断增加。随着CaCO3的加入,气还原铜渣的表观活化能显著提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


