Analytical methods for in-depth characterisation of cell culture bioreactors: A case study
IF 3.2
引用次数: 0
Abstract
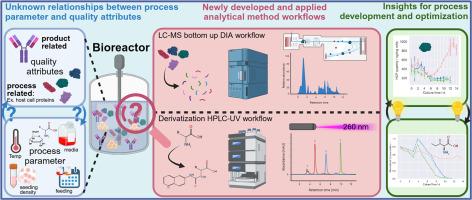
Biopharmaceuticals and especially antibodies represent an expanding segment of pharmaceutical drug products. Production processes for this group of complex molecules use biological expression systems such as Chinese hamster ovary (CHO) cells, that are instrumental in their assembly, folding, and post-translational modification. Process development and optimization is challenging due to the complexity of cell culture systems and interdependency of process parameters on product quality attributes. This case study presents newly applied and developed analytical technologies able to monitor the cell culture process to increase the understanding of the interconnection of process parameters and quality attribute relationships in biopharmaceutical production processes. Specifically, a high-performance liquid chromatography (HPLC) -based workflow routinely measures amino acids and other cell culture media component profiles, while a mass spectrometry-based workflow monitors and quantifies changes in expressions of host cell proteins (HCPs) both globally and for individual HCP during the culture duration. These methods were applied to an industrial process with variations in seeding density, glucose and media feeding strategy, and media composition. Observations on product titre, global HCP and individual high-risk HCPs, throughout the culture progress, as well as in the end of the culture, provide insight for potential further optimization.
深入表征细胞培养生物反应器的分析方法:一个案例研究
生物制药,特别是抗体代表了医药产品的一个不断扩大的部分。这组复杂分子的生产过程使用生物表达系统,如中国仓鼠卵巢(CHO)细胞,这有助于它们的组装,折叠和翻译后修饰。由于细胞培养系统的复杂性和工艺参数对产品质量属性的相互依赖性,工艺开发和优化具有挑战性。本案例研究展示了新应用和开发的分析技术,能够监测细胞培养过程,以增加对生物制药生产过程中工艺参数和质量属性关系的互连的理解。具体来说,基于高效液相色谱(HPLC)的工作流程常规测量氨基酸和其他细胞培养基成分谱,而基于质谱的工作流程监测和量化宿主细胞蛋白(HCP)在培养期间的整体和个体表达变化。这些方法被应用到一个工业过程中,在播种密度,葡萄糖和培养基喂养策略,和培养基组成的变化。在整个培养过程中以及培养结束时,对产品滴度、整体HCP和个体高风险HCP的观察,为进一步优化提供了潜在的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of chromatography open
Analytical Chemistry
CiteScore
2.50
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


