In situ atomic-resolution imaging of water vapor–driven multistep oxidation dynamics in strontium cobaltite
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Understanding how water vapor interacts with transition metal oxides (TMOs) is critical for tailoring material properties to improve performance and enable new technologies. Despite extensive research efforts, atomic-scale mechanisms underpinning dynamic reactions and reaction-induced phase transitions remain elusive. Here, we use in situ environmental transmission electron microscopy to investigate how water vapor oxidizes vacancy-ordered SrCoO2.5 at moderately elevated temperatures, demonstrating that water molecules can initiate oxidation more effectively than oxygen under comparable conditions. We discover a distinct “staging” behavior during the oxidation process: A fully ordered intermediate phase, SrCoO2.75, forms before transitioning into a near-perovskite SrCoO3−δ. In addition, antiphase boundaries, originating at step terraces of SrTiO3, alleviate strain by creating reversible nanoscale “gaps” during lattice contraction under oxidation, providing a pathway for preserving structural integrity throughout redox cycling. This work provides atomic-level guidance for engineering TMOs by leveraging water vapor to control their redox behavior and tailor functional properties.
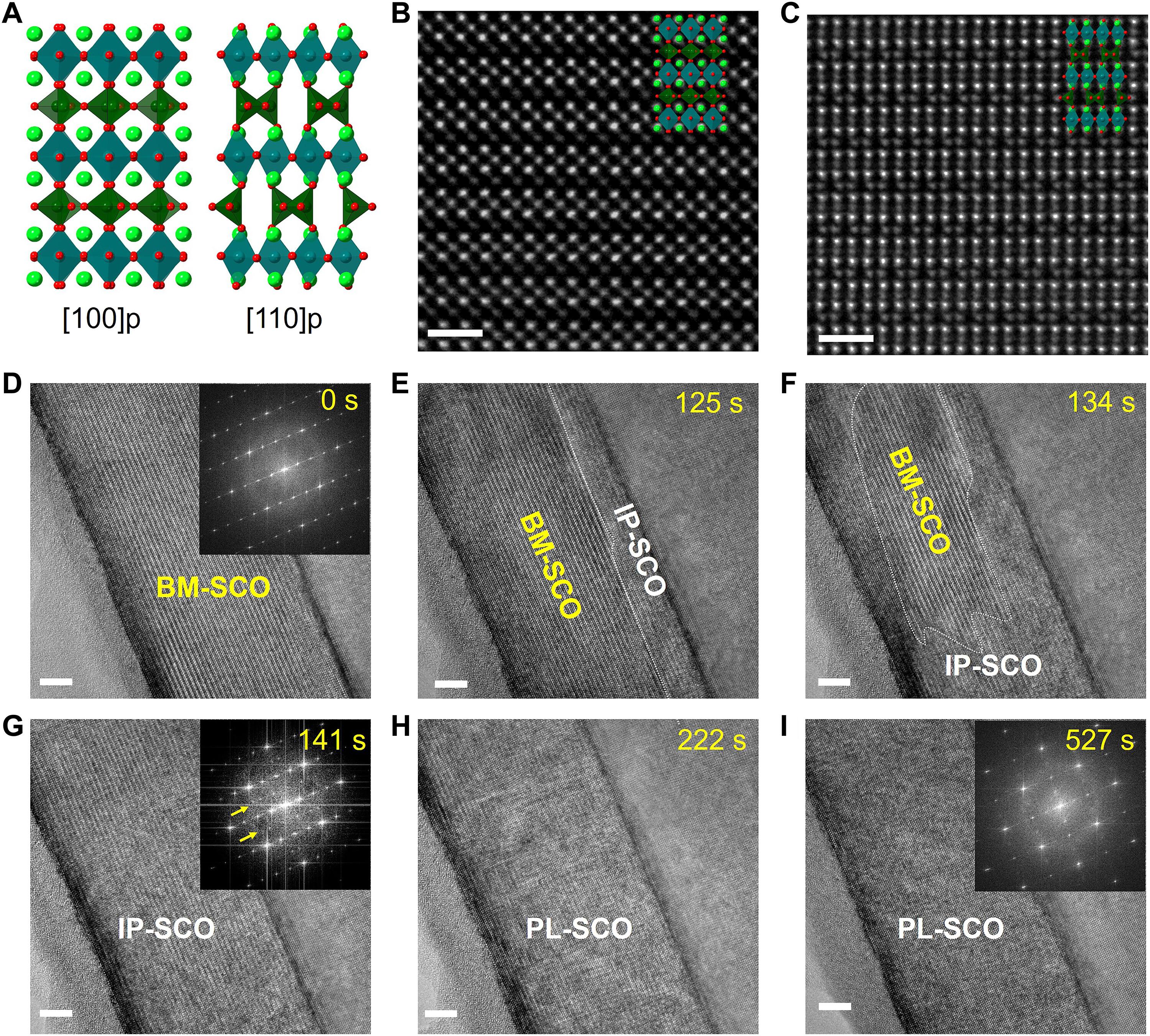
水蒸气驱动的钴酸锶多步氧化动力学的原位原子分辨率成像
了解水蒸气如何与过渡金属氧化物(TMOs)相互作用对于定制材料性能以提高性能和实现新技术至关重要。尽管进行了大量的研究,但支持动态反应和反应诱导相变的原子尺度机制仍然难以捉摸。在这里,我们使用原位环境透射电子显微镜来研究水蒸气如何在中等高温下氧化空位有序的SrCoO2.5,证明在类似条件下,水分子比氧气更有效地引发氧化。在氧化过程中,我们发现了一个明显的“分期”行为:在过渡到近钙钛矿的SrCoO3−δ之前,形成了一个完全有序的中间相SrCoO2.75。此外,反相边界起源于SrTiO3的阶梯式,通过在氧化下晶格收缩过程中产生可逆的纳米级“间隙”来减轻应变,为在氧化还原循环中保持结构完整性提供了一条途径。这项工作为工程TMOs提供了原子水平的指导,通过利用水蒸气来控制其氧化还原行为和定制功能特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


