Adsorption-attraction electrolyte addressing anion-deficient interface for lithium metal batteries
IF 36.6
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
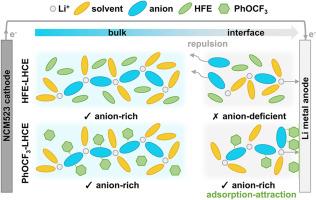
Constructing an optimal solid–electrolyte interphase (SEI) through electrolyte strategies is an effective approach to suppress lithium dendrites and improve deposition/stripping reversibility. Specifically, increasing the proportion of anion coordination in the inner Li+ solvation sheath promotes the formation of an anion-derived SEI that features a high content of inorganic components favoring Li+ diffusion. However, whether this anion-rich structure can persist during cycling has not been dynamically investigated. In this work, we not only construct a favorable solvation structure but also study its evolution in both bulk and interface regions across varying temperatures. Additionally, we employ the unique “adsorption-attraction” mechanism of trifluoromethoxybenzene (PhOCF3) solvent to inhibit the undesirable transition from an “anion-rich” to “anion-deficient” structure at the anode interface, which is confirmed by 2D NMR and in situ infrared spectroscopy. In summary, this work explores the solvation structure in depth and proposes new perspectives on designing electrolytes for lithium metal batteries.
锂金属电池阴离子缺乏界面的吸附-吸引电解质
通过电解质策略构建最佳固-电解质间相(SEI)是抑制锂枝晶和提高沉积/剥离可逆性的有效途径。具体来说,增加内部Li+溶剂化鞘中阴离子配位的比例,促进阴离子衍生SEI的形成,该SEI具有高含量的无机成分,有利于Li+的扩散。然而,这种富含阴离子的结构是否能在循环过程中持续存在尚未得到动态研究。在这项工作中,我们不仅构建了一个有利的溶剂化结构,而且研究了它在不同温度下在体区和界面区的演变。此外,我们利用三氟甲氧基苯(PhOCF3)溶剂独特的“吸附-吸引”机制,抑制了阳极界面从“富阴离子”到“缺阴离子”结构的不良转变,这一点得到了二维核磁共振和原位红外光谱的证实。综上所述,本研究深入探讨了溶剂化结构,为锂金属电池电解质的设计提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


