Immunoinformatics design and experimental expression of a multi-epitope vaccine simultaneously targeting AAV2 and HAdV-F41 against acute hepatitis of unknown etiology
IF 2.4
3区 医学
Q3 VIROLOGY
引用次数: 0
Abstract
The recent global outbreak of acute hepatitis of unknown etiology (AHUE) in children has raised significant health concerns due to the severity of infections, some of which require liver transplants and can lead to fatalities. Emerging evidence suggests that AHUE is caused by a co-infection involving AAV2 and HAdV-F41. Through immunoinformatics, we identified optimal T-cell and B-cell epitopes from AAV2's VP1 and AAP, as well as from HAdV-F41's long fiber, short fiber, and hexon proteins. To enhance specific immune responses, we incorporated the pan DR-binding epitope (PADRE) and Mycobacterium tuberculosis resuscitation-promoting factor RpfE as adjuvants, linking these elements with appropriate linkers to create a multi-epitope vaccine (MEV). The MEV gene was codon-optimized, cloned into the pET-15b vector, expressed in bacterial hosts, and purified using affinity chromatography. The resulting candidate vaccine, MEV-3, demonstrated high antigenicity, non-allergenicity, and non-toxicity, with a low instability index and favorable molecular characteristics. Molecular dynamics simulations confirmed the vaccine's stable binding to immune receptors, and prokaryotic expression yielded stable and pure MEV-3. Computational immune analysis further predicted a strong immune response induced by this vaccine. In conclusion, we developed an MEV that simultaneously targets AAV2 and HAdV-F41, potentially offering effective prevention and treatment for AHUE.
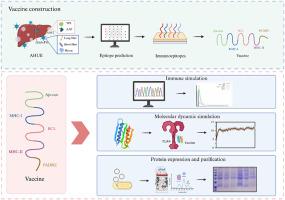
同时靶向AAV2和HAdV-F41治疗不明原因急性肝炎的多表位疫苗的免疫信息学设计和实验表达
由于感染的严重程度,最近全球爆发的不明原因急性儿童肝炎(AHUE)引起了重大的健康问题,其中一些感染需要肝脏移植,并可能导致死亡。新出现的证据表明,AHUE是由AAV2和HAdV-F41共同感染引起的。通过免疫信息学,我们从AAV2的VP1和AAP,以及HAdV-F41的长纤维、短纤维和六邻体蛋白中鉴定出最佳的t细胞和b细胞表位。为了增强特异性免疫应答,我们将泛dr结合表位(PADRE)和结核分枝杆菌复苏促进因子RpfE作为佐剂,将这些元件与适当的连接物连接,以创建多表位疫苗(MEV)。对MEV基因进行密码子优化,克隆到pET-15b载体中,在细菌宿主中表达,并用亲和层析纯化。该候选疫苗MEV-3具有高抗原性、无致敏性、无毒性、低不稳定性指数和良好的分子特性。分子动力学模拟证实了疫苗与免疫受体的稳定结合,原核表达产生了稳定和纯净的MEV-3。计算免疫分析进一步预测了该疫苗诱导的强免疫应答。总之,我们开发了一种同时靶向AAV2和HAdV-F41的MEV,可能为AHUE提供有效的预防和治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Virology
医学-病毒学
CiteScore
6.00
自引率
0.00%
发文量
157
审稿时长
50 days
期刊介绍:
Launched in 1955, Virology is a broad and inclusive journal that welcomes submissions on all aspects of virology including plant, animal, microbial and human viruses. The journal publishes basic research as well as pre-clinical and clinical studies of vaccines, anti-viral drugs and their development, anti-viral therapies, and computational studies of virus infections. Any submission that is of broad interest to the community of virologists/vaccinologists and reporting scientifically accurate and valuable research will be considered for publication, including negative findings and multidisciplinary work.Virology is open to reviews, research manuscripts, short communication, registered reports as well as follow-up manuscripts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


