Evaluating the effects of electrolytes on the interaction forces between alumina surfaces in polyacrylic acid solutions using atomic force microscopy
IF 4.2
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
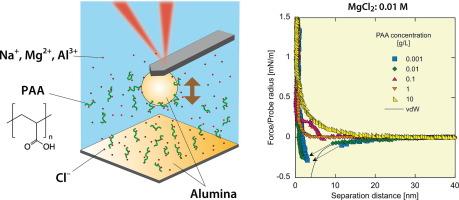
Evaluation and control of ceramic slurry at the microscopic level are critical to ensure consistent quality in manufactured ceramics. Notably, metal ions such as Mg2+ and Al3+ are common in ceramic slurries and significantly influence the stability of particle. This study applied atomic force microscopy to investigate the interaction forces between alumina particle surfaces in the presence of different concentrations of three metal ions and polyacrylic acid (PAA), a widely used dispersant.
The attractive forces observed at low PAA concentrations were attributed to polymer bridging between alumina surfaces, whereas the repulsive forces observed at high PAA concentrations were attributed to the domination of steric repulsion between adsorbed PAA molecules. The presence of multivalent metal ions, such as Mg2+ and Al3+, modulated these interactions; an increasing ion valence induced a transition from repulsive to attractive force, primarily owing to electrostatic screening, which caused conformational collapse of the PAA chains and diminished the range of steric repulsion. Similarly, increasing the concentration of these metal ions decreased the range of repulsive forces, eventually resulting in a net attraction driven by the same electrostatic and polymer conformation mechanisms. Notably, the addition of 0.1 M AlCl3 produced an anomalous long-range attraction between surfaces that could not be explained by conventional mechanisms, such as polymer bridging or electrostatic interactions between charge domains.
用原子力显微镜评价电解质对聚丙烯酸溶液中氧化铝表面相互作用力的影响
陶瓷浆料的微观评价和控制是保证陶瓷制品质量稳定的关键。值得注意的是,Mg2+和Al3+等金属离子在陶瓷浆料中很常见,对颗粒的稳定性有显著影响。本研究应用原子力显微镜研究了氧化铝颗粒表面在不同浓度的三种金属离子和广泛使用的分散剂聚丙烯酸(PAA)存在下的相互作用力。在低PAA浓度下观察到的吸引力归因于氧化铝表面之间的聚合物桥接,而在高PAA浓度下观察到的排斥力归因于吸附PAA分子之间的空间排斥力占主导地位。多价金属离子(如Mg2+和Al3+)的存在调节了这些相互作用;由于静电屏蔽,离子价的增加引起了PAA链的构象崩溃和空间排斥力的减小,从而引起了从排斥力到引力的转变。同样,增加这些金属离子的浓度会减小排斥力的范围,最终产生由相同的静电和聚合物构象机制驱动的净吸引力。值得注意的是,0.1 M AlCl3的加入在表面之间产生了异常的远程吸引力,这无法用传统机制(如聚合物桥接或电荷域之间的静电相互作用)来解释。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Powder Technology
工程技术-工程:化工
CiteScore
9.50
自引率
7.70%
发文量
424
审稿时长
55 days
期刊介绍:
The aim of Advanced Powder Technology is to meet the demand for an international journal that integrates all aspects of science and technology research on powder and particulate materials. The journal fulfills this purpose by publishing original research papers, rapid communications, reviews, and translated articles by prominent researchers worldwide.
The editorial work of Advanced Powder Technology, which was founded as the International Journal of the Society of Powder Technology, Japan, is now shared by distinguished board members, who operate in a unique framework designed to respond to the increasing global demand for articles on not only powder and particles, but also on various materials produced from them.
Advanced Powder Technology covers various areas, but a discussion of powder and particles is required in articles. Topics include: Production of powder and particulate materials in gases and liquids(nanoparticles, fine ceramics, pharmaceuticals, novel functional materials, etc.); Aerosol and colloidal processing; Powder and particle characterization; Dynamics and phenomena; Calculation and simulation (CFD, DEM, Monte Carlo method, population balance, etc.); Measurement and control of powder processes; Particle modification; Comminution; Powder handling and operations (storage, transport, granulation, separation, fluidization, etc.)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


