Composite anion exchange membranes based on poly(biphenyl piperidinium) / ZrO2
IF 3.3
4区 材料科学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The main requirement for the development of Anion Exchange Membranes Fuel Cells (AEMFCs) and Water Electrolyzers (AEMFEs) on an industrial scale is the improvement of Anion Exchange Membranes performance. Besides good ionic conductivity, dimensional stability and mechanical properties in the wet state, the main challenge to be overcome is the improvement of AEMs chemical resistance in harsh alkaline environment. Poly(aryl piperidinium)s are among the most promising AEMs in terms of conductivity, mechanical properties, and chemical stability. Here we report the fabrication and physico-chemical characterization of composite AEMs based on poly(biphenyl piperidinium) (PBP) with the addition of zirconium oxide as a filler to improve membrane properties, including anionic conductivity, water uptake and alkali resistance. The optimal ZrO2 filler content was found to be 5 wt% of dry polymer mass. Compared to plain PBP, composite membranes exhibit increased hydroxide conductivity (from 75 to 116 mS cm−1 at 80 °C), reduced water uptake (from 427 % to 278 % at 80 °C) and swelling ratio (from 85 to 62 % at 80 °C), and a limited reduction (41 %) of cationic groups after ageing in KOH 1 M for 500 h at 80 °C. We demonstrate that ZrO2 filler hinders Hoffman elimination reaction on the piperidinium ring.
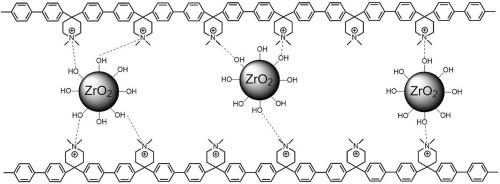
聚联苯哌啶/ ZrO2复合阴离子交换膜
阴离子交换膜燃料电池(aemfc)和水电解槽(AEMFEs)产业化发展的主要要求是提高阴离子交换膜的性能。除了在湿态下具有良好的离子电导率、尺寸稳定性和力学性能外,需要克服的主要挑战是提高AEMs在恶劣碱性环境下的耐化学性。在电导率、机械性能和化学稳定性方面,聚芳基胡椒啶是最有前途的AEMs之一。本文报道了基于聚联苯哌啶(PBP)的复合AEMs的制备和物理化学表征,并添加氧化锆作为填料来改善膜的性能,包括阴离子导电性、吸水性和耐碱性。ZrO2填料的最佳含量为干聚合物质量的5 wt%。与普通PBP相比,复合膜表现出更高的氢氧化物导电性(在80°C时从75到116 mS cm−1),降低的吸水率(在80°C时从427%到278%)和溶胀率(在80°C时从85%到62%),并且在80°C下KOH 1 M中老化500小时后阳离子基团的有限减少(41%)。我们证明了ZrO2填料阻碍了哌啶环上的霍夫曼消去反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Ionics
物理-物理:凝聚态物理
CiteScore
6.10
自引率
3.10%
发文量
152
审稿时长
58 days
期刊介绍:
This interdisciplinary journal is devoted to the physics, chemistry and materials science of diffusion, mass transport, and reactivity of solids. The major part of each issue is devoted to articles on:
(i) physics and chemistry of defects in solids;
(ii) reactions in and on solids, e.g. intercalation, corrosion, oxidation, sintering;
(iii) ion transport measurements, mechanisms and theory;
(iv) solid state electrochemistry;
(v) ionically-electronically mixed conducting solids.
Related technological applications are also included, provided their characteristics are interpreted in terms of the basic solid state properties.
Review papers and relevant symposium proceedings are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


