Construction and optimization of a multienzyme cascade system centered on carbamoyl phosphate synthesis module for efficient guanidinoacetic acid production
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Guanidinoacetic acid (GAA) production is limited by insufficient arginine supply and the accumulation of the byproduct ornithine. Here, we constructed a multi-enzyme cascade centered on carbamoyl phosphate synthesis to enable efficient GAA production through arginine regeneration and byproduct recycling. A structurally guided screen of arginine: glycine amidinotransferase mutants from Arabidopsis thaliana identified the AtE31K/G351N variant, which exhibited a 2.5-fold increase in enzyme activity (7.4 U/mg) and elevated GAA titer from 11.1 mM in wild-type to 15.2 mM. Metabolic flux analysis revealed carbamoyl phosphate as rate-limiting; introducing glutamine synthetase and carbamate kinase enhanced carbamoyl phosphate synthesis and cycle throughput. The eight-enzyme cascade was functionally co-expressed in Escherichia coli BL21 (DE3) through dual-plasmid systems (pRSFDuet-1 and pETDuet-1). After process optimization, 18.35 g/L GAA (156.75 mM) was produced via 60-hour bioconversion with the molar conversion rate of arginine reaching 261.12 %. This work establishes a robust platform for industrial-scale GAA production, and presents a broadly applicable strategy for the rational design and coordinated expression of complex multi-enzyme biosynthetic systems.
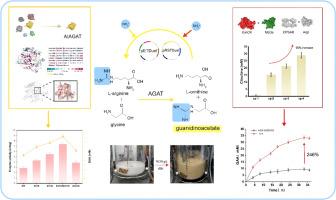
以氨甲酰磷酸合成模块为中心的多酶级联系统的构建与优化,用于高效生产胍基乙酸
胍基乙酸(GAA)的生产受到精氨酸供应不足和副产物鸟氨酸积累的限制。在这里,我们构建了一个以氨甲酰磷酸合成为中心的多酶级联,通过精氨酸再生和副产品回收实现高效的GAA生产。对拟南芥精氨酸:甘氨酸氨基转移酶突变体进行结构引导筛选,鉴定出AtE31K/G351N突变体,其酶活性增加2.5倍(7.4 U/mg), GAA滴度从野生型的11.1 mM提高到15.2 mM。代谢通量分析显示,氨甲酰磷酸是限速的;谷氨酰胺合成酶和氨基甲酸酯激酶的引入提高了磷酸氨基甲酸酯的合成和循环通量。8酶级联通过双质粒系统(pRSFDuet-1和pETDuet-1)在大肠杆菌BL21 (DE3)中功能共表达。经工艺优化,60小时生物转化可制得18.35 g/L的GAA (156.75 mM),精氨酸的摩尔转化率达到261.12%。这项工作为工业规模的GAA生产建立了强大的平台,并为复杂的多酶生物合成系统的合理设计和协调表达提供了广泛适用的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


