Mechanistic insights into monovalent cation selectivity in modified ion selective membranes: A model-based and experimental study
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
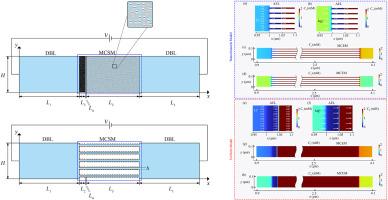
Surface modification techniques that introduce oppositely charged thin layers onto ion exchange membranes have proven highly effective for separating monovalent and divalent ions in chemical and biochemical processes. Although monovalent cation selective membranes (MCSM) have been widely used in electrodialysis, the underlying separation mechanisms and ion transport behavior under varying modification layer charge densities and applied voltages remain insufficiently understood. This study establishes a nanochannel-based MCSM model grounded in the physical nature of porous media, providing clear insights into ion transport behavior and the underlying mechanism of ion selectivity. The model is further validated through electrodialysis experiments. The results show that the nanochannel model captures the influence of membrane pore structure on electric field distribution and ion transport behavior, and reasonably reproduces the experimentally observed trends in selectivity. Near the limiting current density (LC regime), ion transport is primarily governed by diffusion within the modification layer, where the electrostatic barrier more effectively suppresses the migration of divalent Mg2+, leading to enhanced Li/Mg selectivity. More importantly, unlike the uniform model, which predicts a continuous linear increase in selectivity with increasing charge concentration in the modification layer, the nanochannel model reveals a two-stage trend characterized by an initial rise followed by saturation. This nonlinear behavior arises from electrostatic potential saturation near the channel walls caused by counterion accumulation, which restricts further potential propagation and ultimately limits ion selectivity enhancement. These results significantly contribute to our understanding of the mechanisms underpinning the selective transport of monovalent cations and offer valuable guidance for their practical application in selective electrodialysis.
改性离子选择膜中单价阳离子选择性的机理:基于模型和实验的研究
在离子交换膜上引入带相反电荷的薄层的表面改性技术已被证明在化学和生化过程中对分离单价和二价离子非常有效。虽然单价阳离子选择膜(MCSM)已广泛应用于电渗析,但其潜在的分离机制和离子在不同修饰层电荷密度和施加电压下的传输行为仍未得到充分的了解。本研究建立了基于纳米通道的MCSM模型,该模型基于多孔介质的物理性质,为离子传输行为和离子选择性的潜在机制提供了清晰的见解。通过电渗析实验进一步验证了模型的有效性。结果表明,纳米通道模型捕捉到了膜孔结构对电场分布和离子输运行为的影响,并合理地再现了实验观察到的选择性趋势。在极限电流密度(LC区)附近,离子传输主要由修饰层内的扩散控制,其中静电势垒更有效地抑制了二价Mg2+的迁移,导致Li/Mg选择性增强。更重要的是,与均匀模型预测的随修饰层电荷浓度的增加选择性连续线性增加不同,纳米通道模型揭示了一个两阶段的趋势,即最初的上升,然后饱和。这种非线性行为是由反离子积累引起的通道壁附近静电电位饱和引起的,这限制了电位的进一步传播,最终限制了离子选择性的增强。这些结果显著有助于我们理解单价阳离子选择性转运的机制,并为其在选择性电渗析中的实际应用提供了有价值的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Membrane Science
工程技术-高分子科学
CiteScore
17.10
自引率
17.90%
发文量
1031
审稿时长
2.5 months
期刊介绍:
The Journal of Membrane Science is a publication that focuses on membrane systems and is aimed at academic and industrial chemists, chemical engineers, materials scientists, and membranologists. It publishes original research and reviews on various aspects of membrane transport, membrane formation/structure, fouling, module/process design, and processes/applications. The journal primarily focuses on the structure, function, and performance of non-biological membranes but also includes papers that relate to biological membranes. The Journal of Membrane Science publishes Full Text Papers, State-of-the-Art Reviews, Letters to the Editor, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


