Ion liquid selective depolymerization of epoxy resin insulation materials from electric power industry in a polar aprotic solvent system
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
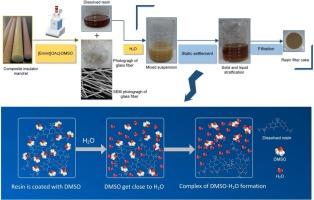
Growing interest has been shown in environmentally friendly, cost-effective methods for recovering and reusing glass fiber-reinforced epoxy resin composites. An efficient strategy was developed for chemical recycling of cured epoxy resin from retired composite insulator mandrel using 1-ethyl-3-methylimidazole acetate ([Emim][OAc])/dimethyl sulfoxide (DMSO) as the depolymerization system. Epoxy resin with chain structure could be recovered with water as precipitant. The process became more affordable and sustainable by eliminating water and then reusing reaction solvent. The resin products' fourier transform infrared spectroscopy (FTIR) and hydrogen nuclear magnetic resonance (HNMR) analysis before and following the decomposition process revealed that the cured crosslink segments (ester group) of epoxy resins could be selectively destroyed. The density functional theory (DFT) illustrated that the solvation effect of DMSO weakened the electrostatic interaction between [OAc]− and [Emim]+, which was significantly more conducive to the nucleophilic addition of imidazole cation. Furthermore, it was proposed to control the selective breaking process of the epoxy resin cured crosslinked ester group by nitrogen heterocyclic carbene reaction, and further esterification to obtain regenerated resin containing ester carbonyl. This recovery process opened up a sustainable path with high atom economy. Retaining of epoxy chain structure was conducive to subsequent secondary curing recovery, and the reaction system could be reused many times.
极性非质子溶剂体系中电力工业用环氧树脂绝缘材料的离子液体选择性解聚
人们对回收和再利用玻璃纤维增强环氧树脂复合材料的环保、经济有效的方法越来越感兴趣。研究了以1-乙基-3-甲基咪唑醋酸酯([Emim][OAc])/二甲基亚砜(DMSO)为解聚体系,对退役复合绝缘子芯筒固化环氧树脂进行化学回收的有效策略。以水为沉淀剂可回收具有链状结构的环氧树脂。通过消除水,然后重复使用反应溶剂,该过程变得更加经济实惠和可持续。分解前后树脂产物的傅里叶红外光谱(FTIR)和氢核磁共振(HNMR)分析表明,固化的环氧树脂交联段(酯基)可以被选择性破坏。密度泛函理论(DFT)表明,DMSO的溶剂化效应减弱了[OAc]−和[Emim]+之间的静电相互作用,更有利于咪唑阳离子的亲核加成。提出通过氮杂环羰基反应控制环氧树脂固化交联酯基的选择性断裂过程,并进一步酯化得到含酯羰基的再生树脂。这一复苏过程开辟了一条高原子经济的可持续发展道路。保留环氧链结构有利于后续的二次固化回收,反应体系可多次重复使用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


