Curing kinetics and surface properties of bio-based polybenzoxazine prepared from monomers synthesized via continuous flow
Abstract
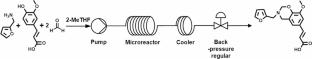
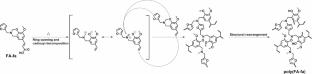
In this work, 2-methyltetrahydrofuran was utilized as a green solvent to synthesize polybenzoxazine precursor (FA-fa) from renewable ferulic acid and furfurylamine in a continuous-flow reactor. The reaction was monitored by the conversion of ferulic acid and the yield of benzoxazine under various conditions, with the optimal reaction temperature and residence time determined using Fourier transform infrared spectroscopy (FTIR) and thermogravimetric analysis. The results show that the conversion of ferulic acid and the yield of FA-fa could reach 84.5% and 75.4%, respectively, at a reaction temperature of 150 °C with an efficient residence time of 12.5 min. The curing kinetics of FA-fa were investigated by in situ FTIR, revealing an apparent activation energy of 155.1 kJ mol-1 and 154.2 kJ mol-1. This relatively low value is the primary reason why FA-fa begins curing at a low temperature of 132.7 °C. The surface free energy of poly(FA-fa) was characterized through contact angle measurements and fully investigated by analyzing the hydrogen bonding constitution via FTIR. The lowest surface free energy was found to be 23.28 mJ·m-2, corresponding to a low content of intermolecular hydrogen bonding constitution with a value of 13.47%.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


