Knockdown of lncRNA NEAT1 suppresses multiple myeloma progression via the miR-133a-3p/ARPC5 axis
IF 2.5
4区 综合性期刊
Q2 MULTIDISCIPLINARY SCIENCES
Journal of Radiation Research and Applied Sciences
Pub Date : 2025-08-22
DOI:10.1016/j.jrras.2025.101878
引用次数: 0
Abstract
Background
Long non-coding RNA nuclear paraspeckle assembly transcript 1 (LncRNA NEAT1) has been implicated in multiple myeloma (MM) pathogenesis, with elevated expression correlating with disease progression and poor clinical outcomes. This study systematically investigated the molecular pathways through which NEAT1 contributes to MM pathogenesis.
Methods
We first characterized NEAT1 expression patterns in MM patients and MM cell lines. Functional studies were performed in NCIH929 and U266 cell lines through gain-of-function (overexpression plasmids) and loss-of-function (small interfering RNA -mediated knockdown) approaches, with assessment of proliferation, invasion and apoptosis. Mechanistic studies employed dual-luciferase reporter assays to validate direct interactions between NEAT1/miR-133a-3p and miR-133a-3p/ARPC5. Rescue experiments elucidated functional relationships within this regulatory axis. Therapeutic potential was evaluated in xenograft models using NEAT1-depleted NCIH929 cells.
Results
Expression analysis revealed that NEAT1 was upregulated 2.2-fold in MM patients (P < 0.01), while miR-133a-3p was downregulated 3.1-fold (P < 0.01) and ARPC5 mRNA levels was increased 2.0-fold (P < 0.01). NEAT1 overexpression notably enhanced MM cell proliferation and invasion, and suppressed apoptosis (P < 0.01). Conversely, NEAT1 knockdown yielded opposite effects (all P < 0.01). Mechanistically, miR-133a-3p was a target gene of NEAT1 and negatively regulated by NEAT1. The inhibition of miR-133a-3p counteracted the tumor-suppressive effect of NEAT1 knockdown on MM cell progression. Furthermore, ARPC5 was validated as a downstream target of miR-133a-3p, and the overexpression of ARPC5 nullified the inhibitory effect of miR-133a-3p overexpression on MM cell progression (P < 0.01). Additionally, NEAT1 knockdown inhibited ARPC5 expression in MM cells (P < 0.01). ARPC5 overexpression abrogated the inhibitory effects of NEAT1 knockdown on MM cell progression (P < 0.01). Additionally, NEAT1 depletion significantly suppressed tumor growth in vivo (P < 0.01).
Conclusion
LncRNA NEAT1 knockdown suppressed proliferation and invasion, and facilitated apoptosis of MM cells via targeting the miR-133a-3p/ARPC5 pathway. This study is the first to demonstrate that NEAT1 promotes MM progression by sponging miR-133a-3p to upregulate ARPC5, revealing a novel therapeutic axis.
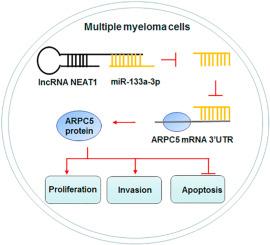
lncRNA NEAT1的敲低通过miR-133a-3p/ARPC5轴抑制多发性骨髓瘤的进展
长链非编码RNA核旁斑组装转录物1 (LncRNA NEAT1)与多发性骨髓瘤(MM)的发病机制有关,其表达升高与疾病进展和不良临床结果相关。本研究系统地探讨了NEAT1参与MM发病的分子途径。方法首先分析NEAT1在MM患者和MM细胞系中的表达模式。通过功能获得(过表达质粒)和功能丧失(小干扰RNA介导的敲低)方法对NCIH929和U266细胞系进行功能研究,并评估其增殖、侵袭和凋亡情况。机制研究采用双荧光素酶报告基因检测来验证NEAT1/miR-133a-3p和miR-133a-3p/ARPC5之间的直接相互作用。救援实验阐明了这一调节轴内的功能关系。使用neat1缺失的NCIH929细胞在异种移植模型中评估治疗潜力。结果NEAT1在MM患者中表达上调2.2倍(P < 0.01), miR-133a-3p下调3.1倍(P < 0.01), ARPC5 mRNA水平升高2.0倍(P < 0.01)。NEAT1过表达可显著增强MM细胞的增殖和侵袭,抑制凋亡(P < 0.01)。相反,NEAT1基因敲低产生相反的效果(P < 0.01)。在机制上,miR-133a-3p是NEAT1的靶基因,受到NEAT1的负调控。miR-133a-3p的抑制抵消了NEAT1敲低对MM细胞进展的肿瘤抑制作用。此外,ARPC5被证实是miR-133a-3p的下游靶点,ARPC5的过表达抵消了miR-133a-3p过表达对MM细胞进展的抑制作用(P < 0.01)。NEAT1敲低可抑制MM细胞中ARPC5的表达(P < 0.01)。ARPC5过表达消除了NEAT1敲低对MM细胞进展的抑制作用(P < 0.01)。此外,NEAT1缺失在体内显著抑制肿瘤生长(P < 0.01)。结论lncrna NEAT1敲低可通过靶向miR-133a-3p/ARPC5通路抑制MM细胞的增殖和侵袭,促进MM细胞凋亡。这项研究首次证明NEAT1通过海绵作用miR-133a-3p上调ARPC5来促进MM的进展,揭示了一种新的治疗轴。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Radiation Research and Applied Sciences
MULTIDISCIPLINARY SCIENCES-
自引率
5.90%
发文量
130
审稿时长
16 weeks
期刊介绍:
Journal of Radiation Research and Applied Sciences provides a high quality medium for the publication of substantial, original and scientific and technological papers on the development and applications of nuclear, radiation and isotopes in biology, medicine, drugs, biochemistry, microbiology, agriculture, entomology, food technology, chemistry, physics, solid states, engineering, environmental and applied sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


