Formation of iodinated disinfection by-products from high-dose disinfection with two types of iodine disinfectant in aquaculture
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Iodinated disinfection by-products (I-DBPs) have raised concerns due to their potential cytotoxicity and genotoxicity compared to chlorine and bromine-based by-products. In aquaculture, high-dose iodine-based disinfectants can react with natural organic matter (NOM) in water, generating significant amounts of I-DBPs that might pose environmental risks. This research explored the reaction characteristics of I-DBPs by using two commonly used iodine-based disinfectants (iodine tincture and Polyvinylpyrrolidone-iodine (PVP–I)) in aquaculture. The results indicated that controlling pH at around 8.0 effectively reduced I-DBP formation. A higher concentration of PVP-I (10 μM) was required to produce triiodomethane (TIM) compared to iodine tincture (2.5 μM), likely due to the slower release of iodine from PVP-I. The concentrations of I-DBPs showed a multiplicative growth trend as the concentrations of the iodine-based disinfectants increased. Humic acid (HA) demonstrated a stronger potential for I-DBP formation than algal-derived organic matter (AOM), including extracellular organic matter (EOM) and intracellular organic matter (IOM), due to its strong electron-withdrawing aromatic structure. While EOM led to a greater variety of I-DBPs, IOM contributed more significantly to TIM formation at higher disinfectant concentrations, probably due to its large molecular size and abundance of reactive carbon atoms. The I-DBP formation patterns in the aquaculture water are similar to those of HA, possibly due to the presence of similar compositional components, such as lignin and proteins. The study provides important insights for optimizing iodine disinfectant use in aquaculture and managing I-DBP risks.
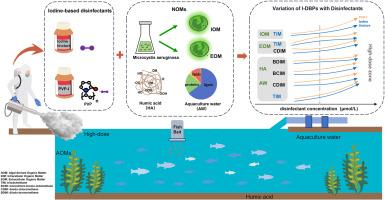
水产养殖中两种碘消毒剂大剂量消毒后碘化消毒副产物的形成
与氯基和溴基副产物相比,碘消毒副产物(I-DBPs)具有潜在的细胞毒性和遗传毒性,因此引起了人们的关注。在水产养殖中,高剂量碘基消毒剂可与水中的天然有机物(NOM)发生反应,产生大量可能构成环境风险的i - dbp。本研究以水产养殖中常用的两种碘基消毒剂(碘酊和聚乙烯吡咯烷酮碘(PVP-I))为研究对象,探讨了I-DBPs的反应特性。结果表明,控制pH在8.0左右可有效减少I-DBP的形成。与碘酊(2.5 μM)相比,产生三碘甲烷(TIM)需要更高浓度的PVP-I (10 μM),这可能是由于PVP-I释放碘的速度较慢。I-DBPs浓度随含碘消毒剂浓度的增加呈倍增增长趋势。腐植酸(HA)具有较强的吸电子芳香结构,比藻类来源的有机物(AOM)(包括胞外有机物(EOM)和胞内有机物(IOM))具有更强的I-DBP形成潜力。虽然EOM导致了更多种类的i - dbp,但在较高的消毒剂浓度下,IOM对TIM的形成贡献更显著,这可能是由于其分子大小较大和活性碳原子的丰度。养殖水体中I-DBP的形成模式与HA相似,可能是由于木质素和蛋白质等相似的组成成分的存在。该研究为优化水产养殖中碘消毒剂的使用和管理I-DBP风险提供了重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


