Hydrophosphonylation strategy to achieve oxazine ring-substituted bio-benzoxazines showing both roles of thermal latent catalyst and flame retardant
IF 7.4
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
The increasing frequent occurrence of fire accidents alone with the desirable sustainable development have accelerated searching for more resilient and safer flame-retardant polymers derived from natural renewable resources. Here we have successfully designed and synthesized bio-based benzoxazine monomers (pH-fa-[2-pH,4-DEP] and pH-fa-[2-oOHph,4-DEP]) with oxazine ring substituted by diethyl phosphite (DEP) via hydrophosphonylation for the first time, thereby achieving non-flammable green thermoset materials. Specifically, pH-fa-[2-oOHph,4-DEP] with phenolic -OH group also shows a latent catalytic feature through its in-built intramolecular hydrogen bonding. As a result, we chose this newly synthesized monomer as both latent catalyst and flame retardant for enhancing the comprehensive properties of the well-commercialized benzoxazine resin (BA-a). The peak curing temperature of BA-a thermosetting system was decreased 23.3 °C and the glass transition temperature of resulting thermoset increased by 61.7 % with only adding 3 mol% of pH-fa-[2-oOHph,4-DEP]. Finally, the mechanistic insights into both advantages including low curing temperature and excellent flame retardancy have been discussed. With this work we demonstrate the possibilities for achieving bio-based non-flammable thermosets using benzoxazine chemistry, and offer molecular level insights into the functions, paving the way for new applications of green thermosetting resins for high-performance non-flammable composite materials.
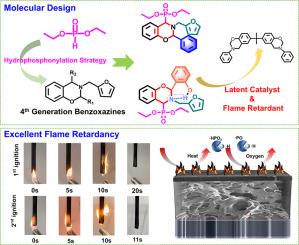
氢膦化策略制备恶嗪环取代生物苯并恶嗪,同时具有热潜催化剂和阻燃剂的作用
随着火灾事故的日益频繁和可持续发展的需要,人们加速了对从自然可再生资源中提取的更有弹性和更安全的阻燃聚合物的探索。本文首次设计合成了以亚磷酸二乙酯(DEP)取代恶嗪环的生物基苯并恶嗪单体(pH-fa-[2-pH,4-DEP]和pH-fa-[2-oOHph,4-DEP]),从而实现了不可燃的绿色热固性材料。具体来说,带有酚- oh基团的pH-fa-[2-oOHph,4-DEP]也通过其内置的分子内氢键表现出潜在的催化特性。因此,我们选择这种新合成的单体作为潜在催化剂和阻燃剂,以提高已商品化的苯并恶嗪树脂(BA-a)的综合性能。当pH-fa-[2-oOHph,4-DEP]加入量为3mol %时,BA-a热固性体系的峰值固化温度降低23.3℃,玻璃化转变温度提高61.7%。最后,讨论了低固化温度和优异阻燃性的机理。通过这项工作,我们展示了使用苯并恶嗪化学实现生物基不可燃热固性的可能性,并提供了分子水平的功能见解,为绿色热固性树脂用于高性能不可燃复合材料的新应用铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Degradation and Stability
化学-高分子科学
CiteScore
10.10
自引率
10.20%
发文量
325
审稿时长
23 days
期刊介绍:
Polymer Degradation and Stability deals with the degradation reactions and their control which are a major preoccupation of practitioners of the many and diverse aspects of modern polymer technology.
Deteriorative reactions occur during processing, when polymers are subjected to heat, oxygen and mechanical stress, and during the useful life of the materials when oxygen and sunlight are the most important degradative agencies. In more specialised applications, degradation may be induced by high energy radiation, ozone, atmospheric pollutants, mechanical stress, biological action, hydrolysis and many other influences. The mechanisms of these reactions and stabilisation processes must be understood if the technology and application of polymers are to continue to advance. The reporting of investigations of this kind is therefore a major function of this journal.
However there are also new developments in polymer technology in which degradation processes find positive applications. For example, photodegradable plastics are now available, the recycling of polymeric products will become increasingly important, degradation and combustion studies are involved in the definition of the fire hazards which are associated with polymeric materials and the microelectronics industry is vitally dependent upon polymer degradation in the manufacture of its circuitry. Polymer properties may also be improved by processes like curing and grafting, the chemistry of which can be closely related to that which causes physical deterioration in other circumstances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


