CO2 hydrogenation to methanol promoted by intermetallic Ni5Ga3 and ZrO2 interface
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Intermetallic alloys have demonstrated significant potential for CO2 hydrogenation to methanol; however, a comprehensive understanding of how the intermetallic-oxide interface regulates the catalytic performance remains elusive. Herein, we reported the influence of interface on the catalytic activity of intermetallic Ni5Ga3 supported on Al2O3 and ZrO2. The Ni5Ga3/ZrO2 interface promoted methanol formation rate by almost one order of magnitude in relation to bare Ni5Ga3. The in-situ spectroscopy combined with theory calculations revealed that the strong intermetallic-oxide interaction promoted the adsorption of H2, facilitating the formation of active hydrogen species. Furthermore, CO2 adsorption was enhanced due to the d-π hybridization between interface Ni sites and CO2 molecules. The reaction predominantly followed the *HCOO pathway to form methanol over the Ni5Ga3/ZrO2 interface, whereas *COOH on the bare intermetallic Ni5Ga3 led to undesired CO. Those findings highlight the critical role of intermetallic-oxides in modulating the reaction pathway in CO2 hydrogenation to methanol.
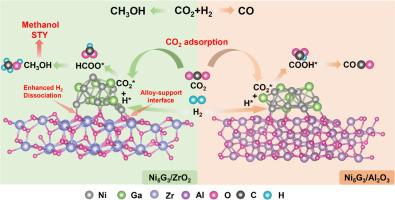
金属间Ni5Ga3和ZrO2界面促进CO2加氢制甲醇
金属间合金已显示出二氧化碳加氢制甲醇的巨大潜力;然而,对金属间氧化物界面如何调节催化性能的全面理解仍然是难以捉摸的。本文报道了界面对负载在Al2O3和ZrO2上的金属间化合物Ni5Ga3催化活性的影响。Ni5Ga3/ZrO2界面与裸Ni5Ga3相比,甲醇的生成速率提高了近一个数量级。原位光谱结合理论计算表明,金属间氧化物之间的强相互作用促进了H2的吸附,有利于活性氢的形成。此外,由于界面Ni位点与CO2分子之间的d-π杂化,CO2吸附增强。该反应主要遵循*HCOO途径在Ni5Ga3/ZrO2界面上生成甲醇,而裸露的金属间Ni5Ga3上的*COOH导致不需要的CO。这些发现突出了金属间氧化物在调节CO2加氢生成甲醇的反应途径中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


