On the deactivation and regeneration mechanisms of Pd/θ-Al2O3 catalysts for propane oxidation
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Pd/Al2O3 is widely used to eliminate hydrocarbon volatile organic compounds (VOCs) via catalytic oxidation under lean conditions. However, a long-standing issue with this catalytic system is the rapid deactivation of the catalyst due to H2O poisoning. In this study, we employed a variety of regeneration methods to treat deactivated Pd/θ-Al2O3, and then conducted detailed characterizations using X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) to explore the underlying mechanisms of catalyst deactivation and regeneration. Our findings indicate that only surface hydroxyls that form on PdO or at the PdO-Al2O3 boundary exhibit poisoning effects. Among the regeneration methods tested, a reduction treatment with H2, followed by in situ or ex situ reoxidation, proved to be the most effective in eliminating these poisoning surface hydroxyls and restoring the highly active form of PdO. In contrast, treatment in O2 at elevated temperatures, although highly efficient in eliminating surface hydroxyls, does not restore the highly active PdO form. This is likely due to adverse high-temperature morphological transformation of PdO that lowers the reactant activation capacity.
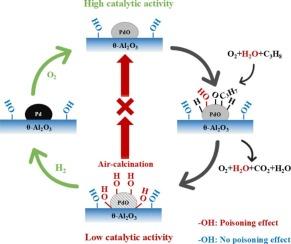
Pd/θ-Al2O3丙烷氧化催化剂的失活与再生机理研究
Pd/Al2O3在稀薄条件下被广泛用于催化氧化去除烃类挥发性有机化合物(VOCs)。然而,这种催化系统的一个长期存在的问题是由于水中毒导致催化剂快速失活。在本研究中,我们采用多种再生方法对失活Pd/θ-Al2O3进行处理,然后利用x射线光电子能谱(XPS)、透射电子显微镜(TEM)和原位漫反射红外傅里叶变换光谱(DRIFTS)进行详细表征,探索催化剂失活和再生的潜在机制。我们的研究结果表明,只有在PdO或PdO- al2o3边界处形成的表面羟基表现出中毒效应。在所测试的再生方法中,H2还原处理,然后原位或非原位再氧化,被证明是最有效的消除这些中毒的表面羟基和恢复高活性形式的PdO。相比之下,在高温O2中处理,虽然在消除表面羟基方面效率很高,但不能恢复高活性的PdO形式。这可能是由于PdO的不利高温形态转变降低了反应物的活化能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


