Blocking Fpr3 ameliorates osteoarthritis by inhibiting NLRP3-mediated chondrocyte pyroptosis
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Objective
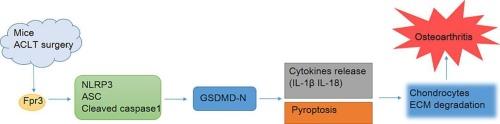
Osteoarthritis (OA) is a chronic joint disorder that frequently diagnosed in elderly individuals. While formyl peptide receptor 3 (Fpr3) is known to play a significant role in inflammation, its involvement in OA progression and chondrocyte pyroptosis remains unclear.
Methods
Anterior cruciate ligament transection (ACLT) was used to create OA mouse model. Lipopolysaccharide (LPS) was utilized to induce inflammation in chondrocytes. We assessed the expression of Fpr3, chondrogenic markers (Collagen-II, SOX9, and Aggrecan), catabolic factors (MMP3, MMP13, and ADAMTS5), inflammatory cytokines (IL-1β, IL-18, and COX-2), and NLRP3 inflammasome members (NLRP3, ASC, Cleaved caspase1, and GSDMD-N) in cartilage from ACLT-induced mouse models and LPS-treated chondrocytes.
Results
Fpr3 expression was significantly increased in both ACLT-induced OA mice and LPS-induced chondrocytes. Fpr3 knockdown reduced chondrocyte injury in OA mice. Fpr3 knockdown promoted the expression of collagen-II, SOX9, and aggrecan, while suppressing the expression of MMP3, MMP13, ADAMTS5, IL-1β, IL-18, COX-2, NLRP3, ASC, cleaved caspase1, and GSDMD-N in both experimental models.
Conclusions
Blocking Fpr3 ameliorated extracellular matrix degradation and pyroptosis during OA progress through the NLRP3 inflammasome. Modulating Fpr3 expression may be a therapeutic target for OA.
阻断Fpr3可通过抑制nlrp3介导的软骨细胞热凋亡改善骨关节炎
目的骨关节炎(OA)是一种常见于老年人的慢性关节疾病。虽然已知甲酰基肽受体3 (Fpr3)在炎症中发挥重要作用,但其在OA进展和软骨细胞焦亡中的作用尚不清楚。方法采用前交叉韧带横断术(ACLT)建立OA小鼠模型。利用脂多糖(LPS)诱导软骨细胞炎症。我们评估了aclt诱导的小鼠模型和lps处理的软骨细胞中Fpr3、软骨生成标志物(Collagen-II、SOX9和Aggrecan)、分解代谢因子(MMP3、MMP13和ADAMTS5)、炎症因子(IL-1β、IL-18和COX-2)和NLRP3炎性体成员(NLRP3、ASC、Cleaved caspase1和GSDMD-N)的表达。结果aclt诱导的OA小鼠和lps诱导的软骨细胞中fpr3的表达均显著升高。Fpr3敲低可减少OA小鼠软骨细胞损伤。Fpr3敲低可促进胶原- ii、SOX9和aggrecan的表达,抑制MMP3、MMP13、ADAMTS5、IL-1β、IL-18、COX-2、NLRP3、ASC、cleaved caspase1和GSDMD-N的表达。结论阻断Fpr3可通过NLRP3炎性体改善OA进展过程中的细胞外基质降解和焦亡。调节Fpr3的表达可能是OA的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


