Targeted mass spectrometry method for the determination of multiple gut-microbiota metabolites in human plasma
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
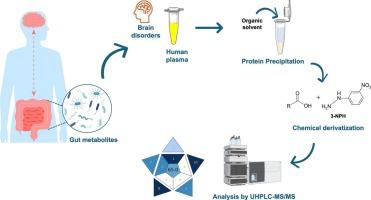
The gut microbiota profoundly impacts human health by producing metabolites that can act as biomarkers for disease diagnosis and therapy. However, accurately measuring these metabolites in biomatrices is challenging due to their low concentrations, high molecular diversity, and interference from matrix components, demanding advanced and precise analytical methodologies. Hence, an ultra-high-performance liquid chromatography method coupled to triple quadrupole-tandem mass spectrometry detection, combined with a chemical derivatization procedure, was developed and validated to quantify seven gut metabolites, namely acetic acid, propionic acid, butyric acid, p-cresol sulfate, 3-indoxyl sulfate, indole-3-acetic acid, and L-tryptophan, in human plasma. Samples were prepared by protein precipitation with acetonitrile and subsequently derivatized using 3-nitrophenylhydrazine. Chromatographic separation was achieved using a BEH C18 column, with elution performed at a flow rate of 0.2 mL min−1 and in gradient mode using formic acid-water (1:1000, v/v) and formic acid-acetonitrile (1:1000, v/v) as mobile phase components. The mass spectrometer was operated in negative ionization mode and data was acquired in selected reaction monitoring. Good linearity was achieved (r2 > 0.997) for all the target gut metabolites in the evaluated concentration ranges, with low LLOQ values (0.4–8 μM). The method proved to be accurate (87.0–114 %) and precise (CV ≤ 13.5 %), achieving a score of 65 in the Blue Applicability Grade Index (BAGI) metric, which confirmed its practicality. The developed method was ultimately employed to the analysis of plasma samples from children and adults involved in clinical studies, demonstrating its usefulness in medical research.
靶向质谱法测定人血浆中多种肠道微生物代谢物
肠道微生物群通过产生代谢物深刻地影响人类健康,代谢物可以作为疾病诊断和治疗的生物标志物。然而,由于生物基质中代谢物的低浓度、高分子多样性和基质成分的干扰,准确测量这些代谢物具有挑战性,需要先进和精确的分析方法。因此,我们建立了一种超高效液相色谱联用三重四极杆串联质谱检测方法,结合化学衍生化程序,用于定量人血浆中的七种肠道代谢物,即乙酸、丙酸、丁酸、对甲酚硫酸盐、3-吲哚酚硫酸盐、吲哚-3-乙酸和l -色氨酸。用乙腈沉淀蛋白质制备样品,然后用3-硝基苯肼衍生化。采用BEH C18色谱柱进行色谱分离,流速为0.2 mL min - 1,以甲酸-水(1:1000,v/v)和甲酸-乙腈(1:1000,v/v)为流动相,梯度模式进行洗脱。质谱仪在负电离模式下工作,并在选定的反应监测中获取数据。在评价浓度范围内,所有目标肠道代谢物均具有良好的线性关系(r2 > 0.997), LLOQ值较低(0.4 ~ 8 μM)。结果表明,该方法具有较高的准确度(87.0 ~ 114%)和精密度(CV≤13.5%),在蓝色适用性等级指数(BAGI)指标中达到65分,证实了该方法的实用性。所开发的方法最终被用于分析参与临床研究的儿童和成人的血浆样本,证明了其在医学研究中的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


