An energy metabolism-engaged nanomedicine maintains mitochondrial homeostasis to alleviate cellular ageing
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Energy restriction is closely related to cellular senescence and species longevity. Here, based on the structure and function of ATP synthase, a key enzyme for energy generation, we develop energy metabolism-engaged nanomedicines (EM-eNMs) to rejuvenate aged stromal/stem cells, and help to prevent skeletal ageing. We show that EM-eNMs infiltrate the mitochondria of aged bone marrow mesenchymal stromal/stem cells (BMMSCs), driving mitochondrial fission, mitophagy, glycolysis and maintaining BMMSC stemness and multifunction. The EM-eNMs directly bind to the ATP synthase and promote mitophagy through induction of the dynamin-related protein 1 (DRP1) gene. Remarkably, EM-eNMs selectively target bone tissues through systemic delivery and significantly reverse osteoporotic bone loss in aged mice by enhancing mitochondrial fission and mitophagy, while simultaneously restoring the stemness and osteogenic potential of aged BMMSCs in situ. Taken together, our findings highlight the potential of the EM-eNMs as a targeted therapy to alleviate cellular senescence and age-related diseases. Through binding mitochondrial ATP synthase, engineered nanomedicines rejuvenate aged bone stem cells and restore osteogenesis, reversing osteoporosis in mice and offering a potential senolytic therapy for skeletal ageing.
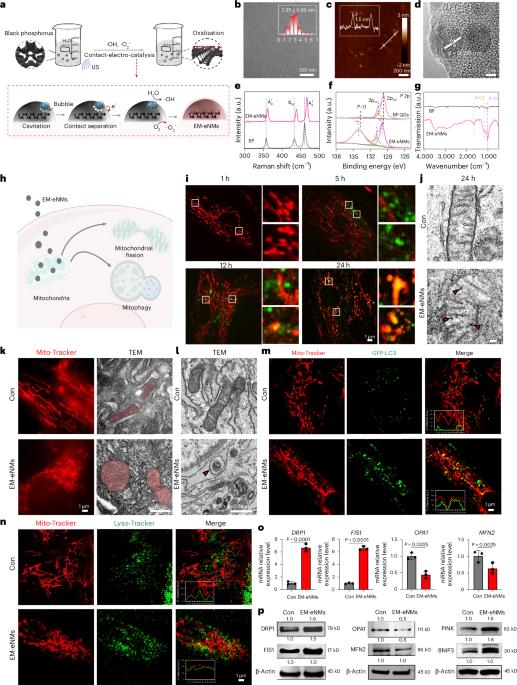
一种能量代谢参与纳米药物维持线粒体稳态,以减轻细胞衰老。
能量限制与细胞衰老和物种寿命密切相关。本研究基于能量生成的关键酶ATP合酶的结构和功能,研究人员开发了能量代谢参与的纳米药物(EM-eNMs),以使老化的基质/干细胞恢复活力,并有助于防止骨骼老化。EM-eNMs可浸润衰老骨髓间充质基质/干细胞(BMMSCs)的线粒体,驱动线粒体分裂、线粒体自噬、糖酵解并维持骨髓间充质干细胞的干性和多功能。EM-eNMs直接与ATP合酶结合,并通过诱导动力蛋白相关蛋白1 (DRP1)基因促进线粒体自噬。值得注意的是,EM-eNMs通过全身递送选择性靶向骨组织,并通过增强线粒体分裂和线粒体自噬显著逆转老年小鼠骨质疏松性骨质流失,同时原位恢复老年bmmsc的干性和成骨潜能。综上所述,我们的研究结果强调了EM-eNMs作为一种靶向治疗来缓解细胞衰老和年龄相关疾病的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


