Paracrine IGFBP3 spatially coordinates IGF signaling to induce myocardial regeneration in mice
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
We hypothesized that the microenvironment of the regenerating neonatal mouse heart contains pro-mitotic factors. To identify non-cell-autonomous effectors of cardiomyocyte mitosis, we analyzed a transcriptomic screen of regenerating and non-regenerating hearts for differentially expressed secreted proteins. We identified IGFBP3 in this screen as a neonatal injury-associated secreted protein. IGFBP3 belongs to a family of proteins that can stabilize and sequester IGF growth factors, as well as exert IGF-independent functions. In the neonatal heart, IGFBP3 is expressed and secreted predominantly by endothelial cells following injury, notably in the border zone of the infarct. We generated loss-of-function and gain-of-function mouse models to dissect the role of IGFBP3 in myocardial regeneration. Global deletion of Igfbp3 blunted neonatal regeneration, while gain-of-function experiments using recombinant IGFBP3 or a tissue-specific ectopic Igfbp3 mouse model uncovered a pro-mitotic effect of IGFBP3 on cardiomyocytes in vitro and in the murine heart. The temporal and spatial expression of an IGFBP3 protease (PAPPA2) and IGFBP3 in the infarct zone suggests that IGFBP3 proteolysis is coordinated to locally release IGF2, which can activate an Insulin/IGF-based growth pathway to stimulate cardiomyocyte division. Collectively, our work illuminates an endothelial-cardiomyocyte crosstalk involving IGFBP3 that can mediate myocardial regeneration in the neonatal heart.
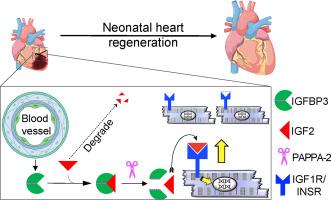
旁分泌IGFBP3空间协调IGF信号诱导小鼠心肌再生。
我们假设新生小鼠心脏再生的微环境中含有促有丝分裂因子。为了鉴定心肌细胞有丝分裂的非细胞自主效应,我们分析了再生和非再生心脏的转录组筛选,以寻找差异表达的分泌蛋白。我们在这个筛选中确定IGFBP3是新生儿损伤相关的分泌蛋白。IGFBP3属于一个稳定和隔离IGF生长因子的蛋白家族,并发挥与IGF无关的功能。在新生儿心脏中,IGFBP3在损伤后主要由内皮细胞表达和分泌,尤其是在梗死边界区。我们建立了功能丧失和功能获得小鼠模型来解剖IGFBP3在心肌再生中的作用。Igfbp3的整体缺失会阻碍新生儿再生,而使用重组Igfbp3或组织特异性异位Igfbp3小鼠模型进行的功能获得实验发现,Igfbp3在体外和小鼠心脏中对心肌细胞具有促有丝分裂作用。IGFBP3蛋白酶(PAPPA2)和IGFBP3在梗死区的时空表达表明,IGFBP3蛋白水解协调局部释放IGF2,从而激活基于胰岛素/ igf的生长通路,刺激心肌细胞分裂。总的来说,我们的工作阐明了涉及IGFBP3的内皮-心肌细胞串扰可以介导新生儿心脏的心肌再生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


