Reticular thalamic hyperexcitability drives autism spectrum disorder behaviors in the Cntnap2 model of autism
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
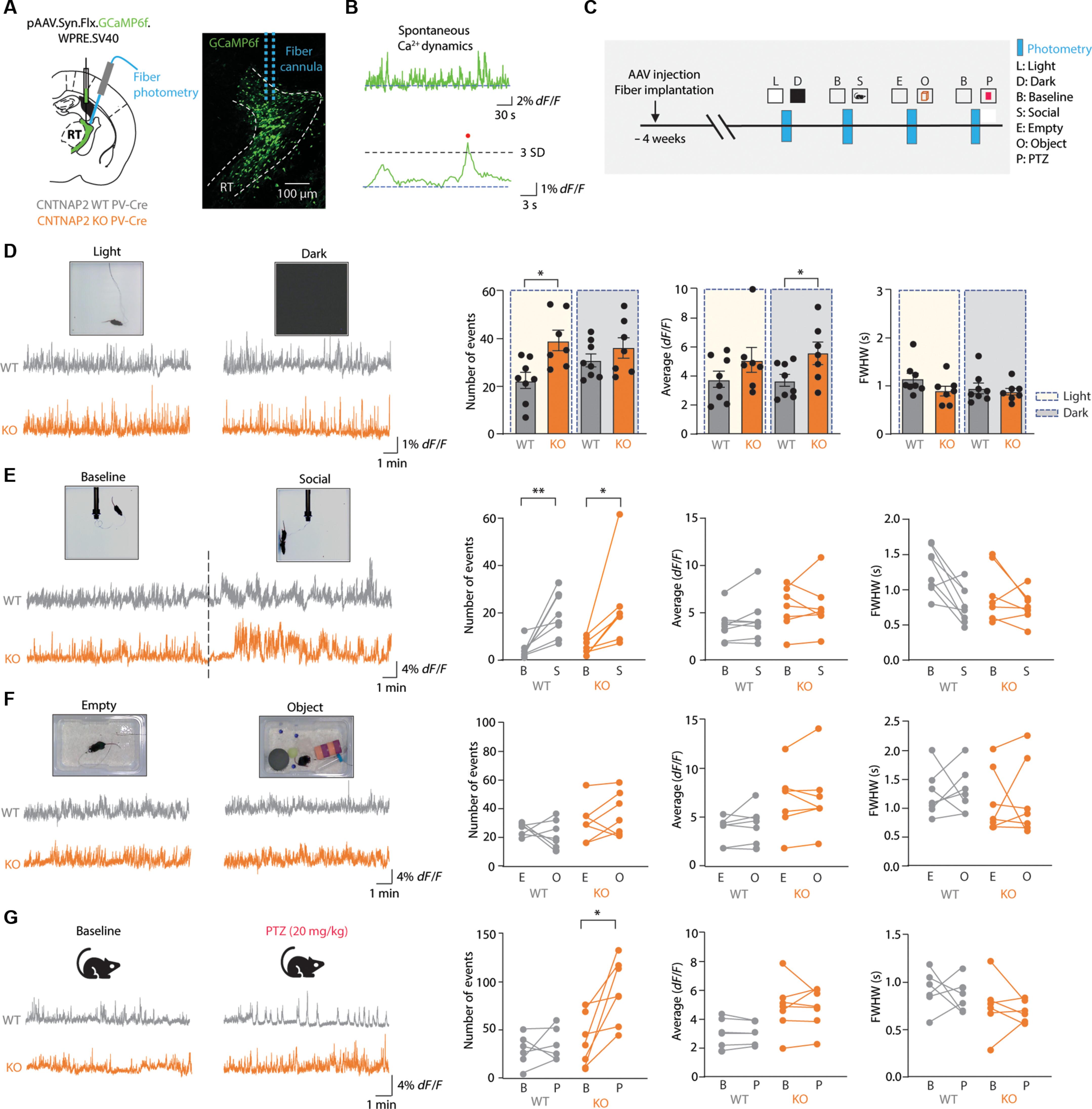
Autism spectrum disorders (ASDs) are neurodevelopmental conditions characterized by social deficits, repetitive behaviors, and comorbidities such as sensory abnormalities, sleep disturbances, and seizures. Although thalamocortical circuit dysfunction has been implicated in these symptoms, its precise roles in ASD pathophysiology remain poorly understood. Here, we examine the specific contribution of the reticular thalamic nucleus (RT), a key modulator of thalamocortical activity, to ASD-related behavioral deficits using a Cntnap2 knockout mouse model. Cntnap2−/− mice displayed increased seizure susceptibility, locomotor activity, and repetitive behaviors. Electrophysiological recordings revealed enhanced intrathalamic oscillations and burst firing in RT neurons, accompanied by elevated T-type calcium currents. In vivo fiber photometry confirmed behavior-associated increases in RT population activity. Notably, pharmacological and chemogenetic suppression of RT excitability via Z944, a T-type calcium channel blocker, and via C21 activation of the inhibitory DREADD hM4Di significantly improved ASD-related behaviors. These findings identify RT hyperexcitability as a mechanistic driver of ASD and highlight RT as a potential therapeutic target.
在Cntnap2自闭症模型中,网状丘脑超兴奋性驱动自闭症谱系障碍行为
自闭症谱系障碍(ASDs)是一种神经发育疾病,其特征是社交缺陷、重复行为和共病,如感觉异常、睡眠障碍和癫痫发作。尽管丘脑皮质回路功能障碍与这些症状有关,但其在ASD病理生理中的确切作用仍知之甚少。在这里,我们使用Cntnap2敲除小鼠模型研究了丘脑网状核(RT)对自闭症相关行为缺陷的具体贡献,RT是丘脑皮质活动的关键调节剂。Cntnap2−/−小鼠表现出增加的癫痫易感性、运动活动和重复行为。电生理记录显示丘脑内振荡增强,RT神经元爆发放电,并伴有t型钙电流升高。体内纤维光度法证实了RT群体活动与行为相关的增加。值得注意的是,通过t型钙通道阻滞剂Z944和C21激活抑制性DREADD hM4Di对RT兴奋性的药理学和化学发生抑制显著改善了asd相关行为。这些发现确定了RT高兴奋性是ASD的机制驱动因素,并强调RT是潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


