Dual-salt electrolyte strategy enables stable interface reaction and high-performance lithium-ion batteries at low temperature
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lithium-ion batteries (LIBs) are increasingly required to operate under harsh conditions, particularly at low-temperature condition. Developing novel electrolytes is a facile and effective approach to elevate the electrochemical performances of LIBs at low temperature. Herein, a dual-salt electrolyte consisting of (lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium difluoro(oxalato)borate (LiODFB)) is proposed to regulate the solvation structure of Li+ ions and improve the reaction kinetics under low temperature. Based on the comprehensive electrochemical tests and theoretical computations, the introduction of LiODFB component not only effectively benefits the formation of cathode electrolyte interface (CEI) layer on the surface of LiFePO4 electrode, but also inhibits the chemical corrosion effect of LiTFSI-containing electrolytes on Al foil. As expected, the optimized Li||LiFePO₄ cells can display high reversible capacity of 117.0 mAh/g after 100 cycles at -20 °C. This work provides both theoretical basis and experimental guidance for the rational design of low-temperature resistant electrolytes.
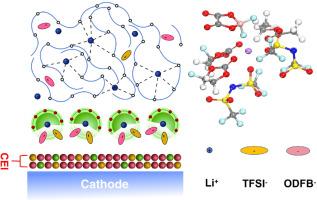
双盐电解质策略可实现稳定的界面反应和低温高性能锂离子电池
锂离子电池(lib)越来越需要在恶劣的条件下工作,特别是在低温条件下。开发新型电解质是提高锂离子电池低温电化学性能的一种简便有效的方法。本文提出了一种由二(三氟甲烷磺酰)亚胺锂(LiTFSI)和二氟(草酸)硼酸锂(LiODFB)组成的双盐电解质,以调节Li+离子的溶剂化结构,改善低温下的反应动力学。综合电化学测试和理论计算表明,LiODFB组分的引入不仅有效地促进了LiFePO4电极表面阴极电解质界面(CEI)层的形成,而且抑制了含litfsi电解质对Al箔的化学腐蚀作用。正如预期的那样,优化后的Li||LiFePO₄电池在-20 °C下循环100次后可以显示出117.0 mAh/g的高可逆容量。该工作为耐低温电解液的合理设计提供了理论依据和实验指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


