Discovery of dopamine D1 receptor antagonists from Stephania epigaea using label-free cell phenotypic assays
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
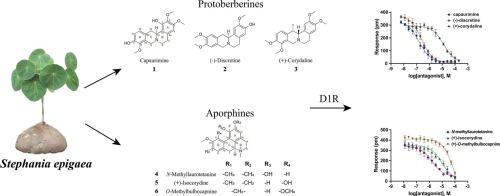
The traditional Chinese medicine Stephania epigaea (S. epigaea) has been used for medicinal purposes, including the treatment of abdominal pain and malaria. Increasing evidence suggests a relationship between pain relief, gut regulation, and the dopaminergic system, particularly the dopamine D1 receptor (D1R). However, it remains unclear whether and how active components of S. epigaea exert their effects through dopamine receptors. This study aims to discover active alkaloids targeting D1R from S. epigaea, evaluate their potencies, and analyze their structure-activity relationships (SARs). Twenty-four alkaloids were screened on an HEK293-D1R cell model using label-free cell phenotypic assays, and six were identified for the first time as exhibiting D1R antagonistic activity, including capaurimine, (−)-discretine, (+)-corydaline, N-methyllaurotetanine, (+)-isocorydine and O-methylbulbocapnine. Among these, protoberberine-type alkaloids capaurimine and (−)-discretine were found to be potent D1R antagonists, with IC50 values of 0.16 ± 0.02 μM and 0.25 ± 0.01 μM, respectively. The aporphine-type alkaloid N-methyllaurotetanine also exhibited micromolar-level D1R antagonistic activity (IC50 = 1.65 ± 0.20 μM). Their kinetic binding profiles were characterized using co-stimulation assay, confirming them as competitive D1R antagonists. Additionally, these compounds were docked with the crystal structure of human D1R, allowing for an analysis their SARs. These findings provide potential mechanisms underlying the analgesic and gastrointestinal effects of S. epigaea and offer valuable information for D1R antagonist drug design.
利用无标记细胞表型分析发现附子藤多巴胺D1受体拮抗剂
中药菝葜(S. epigaea)已被用于医疗目的,包括治疗腹痛和疟疾。越来越多的证据表明疼痛缓解、肠道调节和多巴胺能系统,特别是多巴胺D1受体(D1R)之间存在关系。然而,尚不清楚是否以及如何通过多巴胺受体发挥作用的活性成分。本研究旨在从附属物中发现靶向D1R的活性生物碱,评价其药效,并分析其构效关系(sar)。在HEK293-D1R细胞模型上筛选了24种生物碱,其中6种首次被鉴定出具有D1R拮抗活性,包括capaurimine,(−)- distine, (+)- corydine, N-methyllaurotetanine, (+)-isocorydine和O-methylbulbocapnine。其中,原小檗碱型生物碱capaurimine和(−)-discretine是有效的D1R拮抗剂,IC50值分别为0.16±0.02 μM和0.25±0.01 μM。阿啡型生物碱n -甲基lauro破伤风碱也表现出微摩尔水平的D1R拮抗活性(IC50 = 1.65±0.20 μM)。用共刺激实验对它们的动力学结合谱进行了表征,证实它们是具有竞争性的D1R拮抗剂。此外,这些化合物与人类D1R的晶体结构对接,可以分析它们的SARs。这些发现提供了附着物镇痛和胃肠道作用的潜在机制,并为D1R拮抗剂的药物设计提供了有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


