Synergistic optimization of RuNi alloy thin films via aerosol-assisted chemical vapor deposition for efficient hydrogen evolution in acidic media
IF 7.9
3区 材料科学
Q1 GREEN & SUSTAINABLE SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Ruthenium (Ru)-based catalysts are promising alternatives to platinum (Pt) for the hydrogen evolution reaction (HER) due to their comparable hydrogen adsorption properties and lower cost. Efficient synthesis of Ru catalysts for achieving high current density and stability has become crucial for large-scale hydrogen production via water electrolysis. This work demonstrates that RuNi alloy thin films fabricated through a simple aerosol-assisted chemical vapor deposition process show great promise for HER under acidic conditions. The alloy catalyst, designed with an optimized 1:1 elemental composition, exhibits a granular morphology and facilitates strong synergistic electronic interactions between Ru and Ni. This all leads to develop an outstanding catalytic performance, including a current density of 1 A cm−2 at an overpotential of 152 mV, a low Tafel slope of 38 mV dec−1 and high electrochemical surface area. The robust RuNi thin film maintains continuous HER activity for 45 h at 25 and 50 mA cm−2 without changes in composition or morphology. Density functional theory calculations reveal that Ni weakens hydrogen adsorption on Ru sites and enhances charge transfer between Ru and Ni, which drives the significantly improved HER activity. This work emphasizes fabricating thin-film catalysts via a tailored CVD process to achieve promising water-splitting performance.
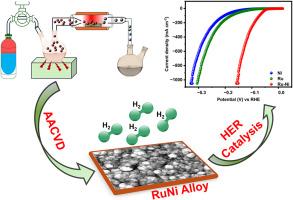
气溶胶辅助化学气相沉积法优化润尼合金薄膜在酸性介质中的高效析氢
钌(Ru)基催化剂由于具有相当的氢吸附性能和较低的成本,是铂(Pt)基催化剂在析氢反应(HER)中很有前途的替代品。高效合成钌催化剂以获得高电流密度和稳定性已成为大规模水电解制氢的关键。这项工作表明,通过简单的气溶胶辅助化学气相沉积工艺制备的RuNi合金薄膜在酸性条件下具有很大的应用前景。设计的合金催化剂具有优化的1:1元素组成,呈现颗粒状形态,有利于Ru和Ni之间的强协同电子相互作用。这一切都导致了优异的催化性能,包括在152 mV过电位下的电流密度为1 a cm−2,低Tafel斜率为38 mV dec−1和高电化学表面积。坚固的RuNi薄膜在25和50 mA cm−2下保持45小时的连续HER活性,而不改变其组成或形态。密度泛函理论计算表明,Ni削弱了氢在Ru位点上的吸附,增强了Ru和Ni之间的电荷转移,从而显著提高了HER活性。这项工作强调通过定制的CVD工艺制造薄膜催化剂,以实现有希望的水分解性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Sustainability
Multiple-
CiteScore
5.80
自引率
6.40%
发文量
174
审稿时长
32 days
期刊介绍:
Materials Today Sustainability is a multi-disciplinary journal covering all aspects of sustainability through materials science.
With a rapidly increasing population with growing demands, materials science has emerged as a critical discipline toward protecting of the environment and ensuring the long term survival of future generations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


