Catalyst layer at the junction of a forward bias bipolar membrane for CO2 electrolysis
IF 4.6
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
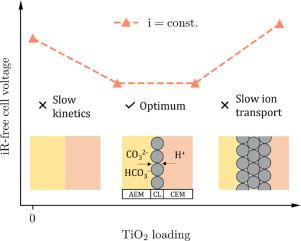
CO2 reduction in an electrolysis cell with a forward bias bipolar membrane (BPM) ensures good selectivity and CO2 utilization, but still suffers from large overvoltages. Recent studies have shown that integrating metal-oxide catalysts at the BPM junction in reverse bias significantly enhances the performance of water electrolyzers. It remains unclear if this method has the same positive effect on CO2 electrolysis. We studied the performance of a specially designed zero-gap BPM CO2 electrolyzer operating in forward bias mode, incorporating metal-oxide nanoparticles at the BPM interface. For TiO2 catalyst, the optimal loading at the BPM junction was between 10 and 30 μg cm-2, resulting in a 75% higher current density for the same iR-free overpotential. Physical characterization using scanning electron microscopy of the catalyst layers revealed that the optimum performance of the CO2 electrolyzer correlates with a complete coverage. SiO2 and IrO2 metal-oxides were also tested at the BPM junction. SiO2 showed comparable performance to TiO2, whereas IrO2 improved the current density by approximately 100% at an iR-free overpotential of 0.7 V compared to the pristine BPM.
二氧化碳电解用正向偏压双极膜连接处的催化剂层
采用正向偏置双极膜(BPM)的电解池中CO2的减少确保了良好的选择性和CO2利用率,但仍然受到大过电压的影响。最近的研究表明,在反偏置的BPM结处集成金属氧化物催化剂可以显著提高水电解槽的性能。目前尚不清楚这种方法是否对二氧化碳电解有同样的积极影响。我们研究了一种特殊设计的零间隙BPM CO2电解槽的性能,该电解槽在BPM界面处加入金属氧化物纳米粒子,工作在正偏压模式下。对于TiO2催化剂,在BPM连接处的最佳负载为10 ~ 30 μg cm-2,在相同的无ir过电位下,电流密度提高了75%。利用扫描电子显微镜对催化剂层进行的物理表征表明,CO2电解槽的最佳性能与完全覆盖有关。在BPM连接处也测试了SiO2和IrO2金属氧化物。SiO2表现出与TiO2相当的性能,而IrO2在无ir过电位为0.7 V时,与原始BPM相比,电流密度提高了约100%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


