An immunocompetent bioengineered human dermal equivalent to recapitulate scar tissue formation in vitro
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
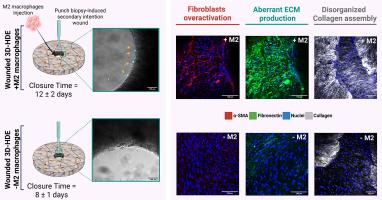
Scar formation, a natural outcome of cutaneous repair, poses significant clinical challenges due to its aesthetic, functional, and psychological impacts. Existing experimental models, while enhancing our understanding of wound healing, often fail to capture the intricate cellular, molecular, and structural mechanisms underlying scar tissue formation. In this study, we present an advanced immunocompetent three-dimensional human dermal equivalent (3D-HDE) model that incorporates M2a macrophages to mimic inflammation and fibrosis in vitro. By inducing secondary intention wounds via punch biopsy and introducing M2a macrophages, the model successfully replicates key features of fibrotic tissue, including the overactivation of fibroblasts into myofibroblasts, aberrant extracellular matrix (ECM) production and dysfunctional ECM assembly. M2a macrophages were found to regulate the anomalous assembly of the ECM, driving the formation of densely packed and aligned collagen type I fibers, primarily by facilitating the transformation of fibroblasts into myofibroblasts. This innovative model provides a physiologically relevant platform for studying the dynamic interplay between macrophages, myofibroblasts, and ECM remodeling during scar evolution. The immunocompetent 3D-HDE model opens new avenues for investigating wound healing mechanisms, fibrosis and the development of targeted anti-fibrotic therapies.
Statement of Significance
Current in vitro tissue models often fail to capture the complexities of human fibrosis at both the cellular and extracellular levels. To address this, our study introduces an immunocompetent three-dimensional human dermal equivalent (3D-HDE) model, incorporating M2 macrophages to explore the role of immune cells in scar tissue formation. This innovative model effectively replicates key features of fibrosis, including fibroblast activation, extracellular matrix (ECM) deposition and remodeling, and collagen alignment. The physiological relevance of our model not only facilitates the study of fibrosis but also enables the testing of anti-fibrotic therapies, providing deeper insights into macrophage-driven wound healing mechanisms. This work bridges a critical gap between traditional in vitro models and clinical applications, advancing our understanding of wound healing, immune responses, and tissue engineering.
一种具有免疫能力的生物工程人皮肤等效物,可在体外重现瘢痕组织形成。
疤痕形成是皮肤修复的自然结果,由于其美学、功能和心理影响,给临床带来了重大挑战。现有的实验模型虽然增强了我们对伤口愈合的理解,但往往无法捕捉到疤痕组织形成背后复杂的细胞、分子和结构机制。在这项研究中,我们提出了一种先进的具有免疫能力的三维人体皮肤等效物(3D-HDE)模型,该模型包含M2a巨噬细胞来模拟体外炎症和纤维化。该模型通过穿刺活检诱导继发性意向伤口,并引入M2a巨噬细胞,成功复制了纤维化组织的关键特征,包括成纤维细胞过度活化成肌成纤维细胞、异常的细胞外基质(ECM)产生和功能失调的ECM组装。研究发现,M2a巨噬细胞主要通过促进成纤维细胞向肌成纤维细胞的转化,调节ECM的异常组装,驱动密集排列的I型胶原纤维的形成。这个创新的模型为研究疤痕进化过程中巨噬细胞、肌成纤维细胞和ECM重塑之间的动态相互作用提供了一个生理学相关的平台。免疫活性3D-HDE模型为研究伤口愈合机制、纤维化和靶向抗纤维化治疗的发展开辟了新的途径。意义声明:目前的体外组织模型往往不能在细胞和细胞外水平捕捉人类纤维化的复杂性。为了解决这个问题,我们的研究引入了一种免疫功能三维人皮肤当量(3D-HDE)模型,结合M2巨噬细胞来探索免疫细胞在疤痕组织形成中的作用。这种创新模型有效地复制了纤维化的关键特征,包括成纤维细胞活化、细胞外基质(ECM)沉积和重塑以及胶原蛋白排列。我们的模型的生理学相关性不仅促进了纤维化的研究,而且使抗纤维化疗法的测试成为可能,为巨噬细胞驱动的伤口愈合机制提供了更深入的见解。这项工作弥合了传统体外模型和临床应用之间的关键差距,促进了我们对伤口愈合、免疫反应和组织工程的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


