Proteogenomic signature of Alzheimer’s disease and related dementia risk in individuals with major depressive disorder
IF 8.7
引用次数: 0
Abstract
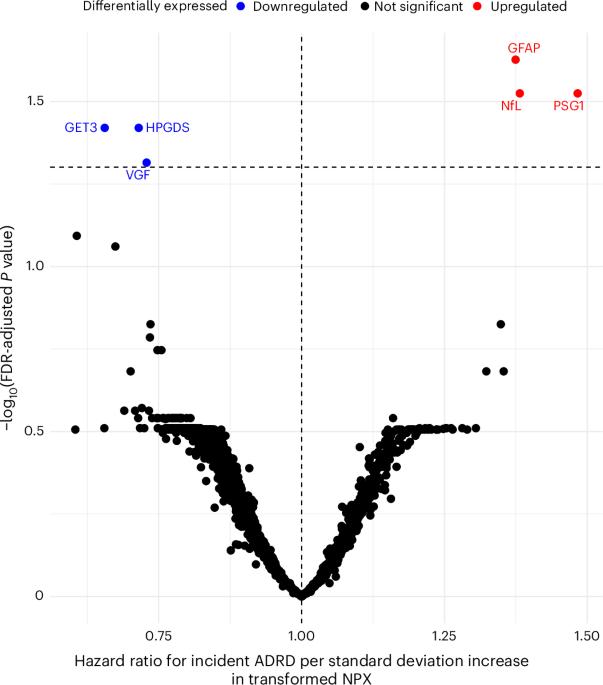
The mechanisms linking a history of major depressive disorder (MDD) to an increased risk of Alzheimer’s disease and related dementia (ADRD) are not fully understood. Using the UK Biobank, we evaluated the biological mechanisms linking the conditions. In participants without history of MDD, 493 proteins were significantly associated with the risk of ADRD. By contrast, in participants with a history of MDD at baseline, a smaller set of six proteins were significantly associated with ADRD risk (NfL, GFAP, PSG1, VGF, GET3 and HPGDS), with GET3 being specifically associated with ADRD risk in the latter group. Two-sample Mendelian randomization analysis showed that the apolipoprotein E and IL-10 receptor subunit B genes were causally linked to incident ADRD. Finally, we developed a proteomic risk score (PrRSMDD-ADRD), which showed strong discriminative power (C statistic = 0.84) to identify participants with MDD who developed ADRD on follow-up. Here we show that plasma proteins associated with inflammation and amyloid-β metabolism are causally linked to a higher ADRD risk in individuals with MDD. Moreover, the PrRSMDD-ADRD can be useful to identify individuals with the highest risk of developing ADRD in a highly vulnerable population. This study investigates biological mechanisms connecting major depressive disorder to Alzheimer’s disease risk, identifying six key proteins and establishing a proteomic risk score with strong predictive power for identifying vulnerable individuals at risk of developing dementia.
重度抑郁症患者阿尔茨海默病和相关痴呆风险的蛋白质基因组特征
重度抑郁症(MDD)病史与阿尔茨海默病及相关痴呆(ADRD)风险增加之间的联系机制尚不完全清楚。使用英国生物银行,我们评估了连接这两种情况的生物学机制。在没有MDD病史的参与者中,493蛋白与ADRD的风险显著相关。相比之下,在基线时有MDD病史的参与者中,一组较小的6种蛋白质(NfL, GFAP, PSG1)与ADRD风险显著相关。VGF, GET3和HPGDS),其中GET3与后一组的ADRD风险特别相关。双样本孟德尔随机化分析表明,APOE和IL-10受体亚单位B基因与ADRD的发生有因果关系。最后,我们开发了一种蛋白质组学风险评分(PrRSMDD-ADRD),该评分具有很强的判别能力(c -统计量= 0.84),可以识别随访中出现ADRD的MDD参与者。本研究表明,与炎症和淀粉样蛋白-β代谢相关的血浆蛋白与重度抑郁症患者较高的ADRD风险有因果关系。此外,PrRSMDD-ADRD可用于识别高危人群中患ADRD风险最高的个体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


