Loss of YTHDF2 enhances Th9 programming and CAR-Th9 cell antitumor efficacy
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
CD4+ T cells differentiate into various subsets, including T helper 1 (Th1), Th2, Th9, Th17 and regulatory T (Treg) cells, which are essential for immune responses and cancer immunotherapy. However, the role of RNA N6-methyladenosine (m6A) modification in this differentiation is unclear. Here we show that YTHDF2, an important m6A reader protein known to destabilize m6A-modified mRNA, negatively regulates Th9 cell differentiation. Ablation of Ythdf2 in both mouse and human naive CD4+ T cells promotes Th9 differentiation by stabilizing Gata3 and Smad3 mRNA under interleukin-4 (IL-4) and transforming growth factor β (TGF-β) signaling, respectively. Ythdf2-deficient Th9 cells produce increased amounts of IL-9 and IL-21, leading to increased tumor infiltration and cytotoxicity by CD8+ T cells and natural killer (NK) cells, thereby improving antitumor activity compared with wild-type Th9 cells. Moreover, YTHDF2 depletion in CAR-Th9 cells enhances their immune activation, reduces their terminal differentiation and augments their antitumor efficacy. Targeting YTHDF2 is thereby a promising strategy to enhance Th9 and CAR-Th9 cell-based cancer immunotherapies. The authors show that the m6A reader protein YTHDF2 negatively regulates Th9 cell differentiation and function. Ablation of YTHDF2 promotes antigen-specific Th9 cell and CAR-Th9 cell antitumor activity in solid tumors.

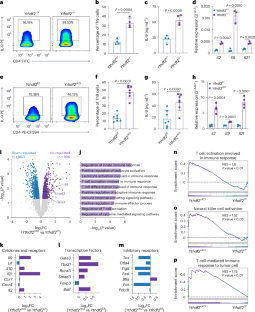
YTHDF2缺失可增强Th9编程和CAR-Th9细胞抗肿瘤效果。
CD4+ T细胞分化为不同的亚群,包括辅助性T细胞1 (Th1)、Th2、Th9、Th17和调节性T细胞(Treg),它们对免疫反应和癌症免疫治疗至关重要。然而,RNA n6 -甲基腺苷(m6A)修饰在这种分化中的作用尚不清楚。在这里,我们发现YTHDF2是一种重要的m6A解读蛋白,已知可以破坏m6A修饰的mRNA的稳定性,并负向调节Th9细胞的分化。消融小鼠和人初始CD4+ T细胞中的Ythdf2分别通过稳定白细胞介素-4 (IL-4)和转化生长因子β (TGF-β)信号传导下的Gata3和Smad3 mRNA来促进Th9的分化。缺乏ythdf2的Th9细胞产生IL-9和IL-21的量增加,导致CD8+ T细胞和自然杀伤(NK)细胞对肿瘤的浸润和细胞毒性增加,从而与野生型Th9细胞相比,抗肿瘤活性提高。此外,CAR-Th9细胞中YTHDF2的缺失增强了其免疫激活,降低了其终末分化,增强了其抗肿瘤功效。因此,靶向YTHDF2是增强Th9和CAR-Th9细胞为基础的癌症免疫治疗的一种有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


