Naringenin prevents ethanol-induced reward behavior in adolescent rats through inhibition of TRPM3-dependent inflammation in the pVTA
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ethanol intake activates the posterior ventral tegmental area (pVTA), enhancing dopamine synthesis in the nucleus accumbens shell (AcbSh) and contributing to Alcohol Use Disorder (AUD). Chronic adolescent ethanol exposure promotes neuroinflammation and disrupts reward pathways, increasing susceptibility to addiction. Although transient receptor potential melastatin 3 (TRPM3) activation promotes inflammation and contributes to psychiatric disorders, its role in reward mechanisms remains unclear. Current AUD treatments reduce cravings and promote alcohol aversion but are often limited by adverse effects, highlighting the need for safer, natural alternatives. Naringenin, a citrus flavonoid, exhibits anti-inflammatory and neuroprotective properties. This study investigates the involvement of TRPM3 in reward processing and whether naringenin modulates its activity to reduce ethanol-induced neuroinflammation and craving. The effect of intraperitoneal (i.p.) naringenin (50 mg/kg) was tested by administering it daily prior to ethanol (2 g/kg, 20% w/v, i.p.) in adolescent male Sprague-Dawley rats for 2 weeks. Reward behavior was assessed using the conditioned place preference and open field tests. The pVTA was examined for TRPM3 expression, biochemical and synaptic changes, while dopamine levels in the AcbSh. Ethanol increases preference scores (0.46±0.01), locomotion (84.4±2.13), TRPM3 expression (48.77±2.58%) and co-expression with tyrosine hydroxylase (27.70±1.29%), TNF-α (298.29±10.48 pg/mg protein) and IL-6 (198.29±9.31 pg/mg protein), dendritic length in the pVTA (887.31±21.07 µm), and dopamine contents in the AcbSh (387,126.1±10,390.04 AUC). Naringenin reversed these effects, suggesting its potential anti-addictive properties and supporting TRPM3 as a promising therapeutic target for AUD.
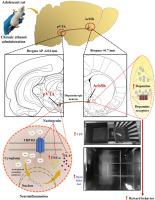
柚皮素通过抑制pVTA中trpm3依赖性炎症来预防青春期大鼠乙醇诱导的奖励行为。
酒精摄入激活后腹侧被覆区(pVTA),增强伏隔核壳(AcbSh)的多巴胺合成,并导致酒精使用障碍(AUD)。青少年长期接触乙醇会促进神经炎症,破坏奖赏通路,增加成瘾的易感性。虽然短暂受体电位美拉他汀3 (TRPM3)激活促进炎症并有助于精神疾病,但其在奖励机制中的作用尚不清楚。目前的AUD治疗方法减少了对酒精的渴望,促进了对酒精的厌恶,但往往受到副作用的限制,这突出了对更安全、天然替代品的需求。柚皮素是一种柑橘类黄酮,具有抗炎和神经保护作用。本研究探讨了TRPM3在奖赏加工中的作用,以及柚皮素是否调节其活性以减少乙醇诱导的神经炎症和渴望。在每日乙醇(2 g/kg, 20% w/v, i.p.)前给药,观察柚皮素[50 mg/kg,腹腔(i.p.)]对青春期雄性Sprague-Dawley大鼠14天的影响。奖励行为评估采用条件位置偏好和开放现场测试。pVTA检测TRPM3表达、生化和突触变化,AcbSh检测多巴胺水平。乙醇增加偏好得分(0.46 ±0.011 )、运动(84.4 ±2.13 ),TRPM3表达式(48.77 ± 2.58%)和co-expression酪氨酸羟化酶(27.70 ± 1.29%),肿瘤坏死因子-α(298.29 ±10.48 pg /毫克蛋白)和il - 6(198.29 ±9.31 pg /毫克蛋白),在pVTA树突长度(887.31 ±21.07 µm),和多巴胺含量AcbSh(387126。1 ± 10390 .04点AUC)。柚皮素逆转了这些作用,表明其潜在的抗成瘾特性,并支持TRPM3作为AUD的有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


