Ketogenic diet influences the renin-angiotensin-aldosterone system components in the healthy and inflamed intestine of male mice
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Inhibiting the overexpression of the renin-angiotensin-aldosterone system (RAAS) alleviates intestinal inflammation. Recently, we and others reported that a high-fat, low carbohydrate, ketogenic diet (KD), shown to downregulate the conventional RAAS components in rat lung and adipose tissue, can protect mice from experimental colitis. Here we assessed whether the proinflammatory angiotensin-converting enzyme - angiotensin receptor type 1 (ACE-AT1R) axis and the anti-inflammatory angiotensin-converting enzyme 2- MAS1 receptor (ACE2-MAS1) axis RAAS components are influenced by the consumption of a KD rich either in saturated fatty acids (SFA-KD) or polyunsaturated linoleic acid (LA-KD) in healthy and inflamed intestine of C57BL/6 J male mice. In healthy jejunum, KD increased the AT2R protein level and decreased Ace2 level regardless of the fat source, whereas in the healthy colon, the RAAS components were unaffected by the dietary interventions. In colon, administration of 2.5% (w/v) dextran sodium sulfate (DSS) for 5 days upregulated ACE protein while downregulating Agtr2 gene expression. These DSS-induced changes were absent in both KD groups. Furthermore, the DSS-SFA-KD group exhibited lower angiotensinogen gene expression than the DSS animals. Additionally, LA-KD mitigated the DSS-induced decrease in Ace2 gene expression. In conclusion, intestinal RAAS component expression is influenced by KDs, and the DSS-induced upregulation of proinflammatory RAAS components were not observed in DSS-KD groups.
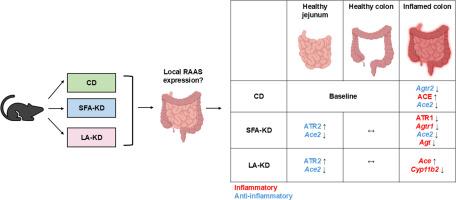
生酮饮食影响健康和炎症雄性小鼠肠道肾素-血管紧张素-醛固酮系统成分。
抑制肾素-血管紧张素-醛固酮系统(RAAS)的过度表达可减轻肠道炎症。最近,我们和其他人报道了高脂肪,低碳水化合物,生酮饮食(KD),显示下调大鼠肺和脂肪组织中的常规RAAS成分,可以保护小鼠免受实验性结肠炎。在这里,我们评估了促炎血管紧张素转换酶-血管紧张素受体1型(ACE-AT1R)轴和抗炎血管紧张素转换酶2-MAS1受体(ACE2-MAS1)轴RAAS组分是否受到C57BL/6J雄性小鼠健康和炎症肠道中富含饱和脂肪酸(SFA-KD)或多不饱和亚油酸(LA-KD)的KD消耗的影响。在健康的空肠中,KD增加了AT2R蛋白水平,降低了Ace2水平,而在健康的结肠中,RAAS成分不受饮食干预的影响。在结肠中,给予2.5% (w/v)葡聚糖硫酸钠(DSS) 5天,ACE蛋白上调,Agtr2基因表达下调。这些dss诱导的变化在两个KD组中都不存在。此外,DSS- sfa - kd组血管紧张素原基因表达低于DSS动物。此外,LA-KD还能减轻dss诱导的Ace2基因表达下降。综上所述,肠道RAAS组分的表达受KDs的影响,DSS-KD组未观察到dss诱导的促炎RAAS组分的上调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


