POU2F1 facilitates the malignant phenotypes and aerobic glycolysis of pituitary adenoma by activating LDHA transcription
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Background
Pituitary adenoma (PA) can be benign, invasive, and metastatic, and its invasiveness and metastasis are poor prognosis factors for PA patients. Lactate dehydrogenase A (LDHA) plays a key role in glycolysis, and its overexpression can promote PA cell proliferation and invasion; however, the underlying mechanisms remain poorly understood.
Methods
Reverse-transcription quantitative polymerase chain reaction and western blot were used to quantify the expression of target genes. MTT, EdU, and flow cytometry assays were utilized to detect the viability, proliferation, and apoptotic rates of HP75 cells, respectively. The invasion and migration of HP75 cells were measured by invasion and wound healing assays, and the sphere formation ability of HP75 cells was analyzed by sphere formation assay. The aerobic glycolysis of HP75 cells was tested by the corresponding kits. The JASPAR, GeneCards, and PROMO databases were used to predict the potential transcription factors of LDHA. The interaction between POU domain class 2 transcription factor 1 (POU2F1) and LDHA was verified by dual-luciferase reporter and chromatin immunoprecipitation assays. A xenograft tumor model was constructed to measure the effects of the POU2F1/LDHA axis on tumor growth in vivo.
Results
LDHA was upregulated in PA tissues, and its expression levels in invasive PA tissues were higher than those in non-invasive ones. LDHA knockdown inhibited the proliferation, invasion, migration, sphere formation and aerobic glycolysis of HP75 cells and induced cell apoptosis. Mechanistically, POU2F1 activated LDHA expression to promote the proliferation, invasion, migration, sphere formation and aerobic glycolysis of HP75 cells and to inhibit cell apoptosis. Besides, LDHA overexpression could ameliorate the PI3K/AKT pathway inactivation induced by POU2F1 silence in vivo and in vitro. LDHA overexpression also alleviated the POU2F1 knockdown-induced inhibitory effect on tumor growth in vivo.
Conclusion
POU2F1-LDHA axis is up-regulated in pituitary adenomas and represents a potential therapeutic target for this disease.
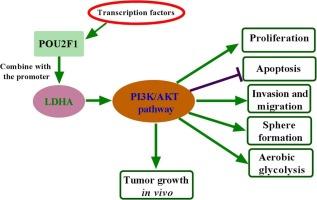
POU2F1通过激活LDHA转录促进垂体腺瘤的恶性表型和有氧糖酵解。
背景:垂体腺瘤(PA)具有良性、侵袭性和转移性,其侵袭性和转移性是影响PA患者预后的不良因素。乳酸脱氢酶A (LDHA)在糖酵解过程中起关键作用,其过表达可促进PA细胞增殖和侵袭;然而,潜在的机制仍然知之甚少。方法:采用逆转录定量聚合酶链反应和western blot技术对靶基因的表达进行定量分析。采用MTT、EdU和流式细胞术分别检测HP75细胞的活力、增殖和凋亡率。采用侵袭法和创面愈合法检测HP75细胞的侵袭和迁移,采用成球法检测HP75细胞的成球能力。采用相应的试剂盒检测HP75细胞的有氧糖酵解。使用JASPAR、GeneCards和PROMO数据库预测LDHA的潜在转录因子。POU结构域2类转录因子1 (POU2F1)与LDHA之间的相互作用通过双荧光素酶报告基因和染色质免疫沉淀实验得到证实。建立异种移植肿瘤模型,测定POU2F1/LDHA轴对肿瘤体内生长的影响。结果:LDHA在PA组织中表达上调,且有创PA组织中LDHA表达水平高于无创PA组织。LDHA敲低抑制HP75细胞的增殖、侵袭、迁移、成球和有氧糖酵解,诱导细胞凋亡。机制上,POU2F1激活LDHA表达,促进HP75细胞的增殖、侵袭、迁移、成球和有氧糖酵解,抑制细胞凋亡。此外,在体内和体外实验中,LDHA过表达可改善POU2F1沉默诱导的PI3K/AKT通路失活。在体内,LDHA过表达也能减轻POU2F1敲低诱导的肿瘤生长抑制作用。结论:POU2F1-LDHA轴在垂体腺瘤中表达上调,是垂体腺瘤的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


