Pesticide-induced epigenetic suppression of WNT signaling and NF-κB-driven inflammation impairs ovarian function in rats
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
The study examined the impact of chlorpyrifos, dimethoate, and their co-exposure on ovarian structure and function in female Wistar rats. The study involved 24 rats, divided into four groups: control, chlorpyrifos (3 mg/kg), dimethoate (30 mg/kg), and combination. Histopathology revealed degenerated and atretic follicles, disorganized granulosa cells and cystic follicles in pesticide exposed rats. Hormonal analysis showed decrease in FSH, LH, PG, T and AMH and increase in ED levels. Gene expression studies revealed significant upregulation of ESR2 (∼2, ∼1.8, and ∼1.85 fold respectively) in chlorpyrifos, dimethoate and combination groups. In contrast, RSPO2, WNT7A, WNT3A and WNT5A were downregulated (reduced by ∼1.73–1.8, 1.7–2.6, 1.45–2.13 and 1.3–1.75 fold, respectively). The changes correlated with reduced β-catenin activation. DNA methylation analysis revealed an inverse correlation between methylation and gene expression, alongside upregulation of DNMT3A and DNMT3B (∼4.5, ∼2.1, ∼4.9; ∼3.75, ∼2.2, ∼3.8 fold in chlorpyrifos, dimethoate and combination groups respectively), suggesting methylation-mediated repression. Furthermore, enhanced expression of inflammation-related genes and cytokines, coupled with NF-κB activation, indicated significant inflammatory responses. Overall, the findings highlight gene specific DNA methylation and inflammatory disruptions in pesticide-exposed ovaries. However, the lack of ChIP assay limits confirmation of DNMT recruitment, which should be addressed in future studies.
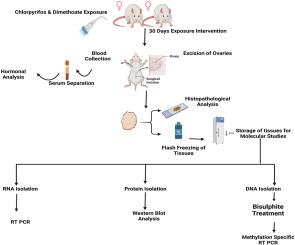
农药诱导的表观遗传抑制WNT信号和NF-κ b驱动的炎症损害大鼠卵巢功能。
本研究考察了毒死蜱、乐果及其共同暴露对雌性Wistar大鼠卵巢结构和功能的影响。该研究涉及24只大鼠,分为四组:对照组、毒死蜱(3mg /kg)组、乐果(30mg /kg)组和复方组。组织病理学检查显示农药暴露大鼠滤泡变性、闭锁、颗粒细胞紊乱、囊性滤泡。激素分析显示FSH、LH、PG、T和AMH降低,ED升高。基因表达研究显示,毒死蜱、乐果和联合用药组ESR2显著上调(分别为~ 2倍、~ 1.8倍和~ 1.85倍)。相比之下,RSPO2、WNT7A、WNT3A和WNT5A下调(分别降低了1.73-1.8倍、1.7-2.6倍、1.45-2.13倍和1.3-1.75倍)。这种变化与β-连环蛋白活性降低有关。DNA甲基化分析显示,甲基化与基因表达呈负相关,同时DNMT3A和DNMT3B上调(毒死蜱、乐果和联合组分别为~ 4.5倍、~ 2.1倍、~ 4.9倍;~ 3.75倍、~ 2.2倍、~ 3.8倍),表明甲基化介导的抑制。此外,炎症相关基因和细胞因子的表达增强,加上NF-κB的激活,表明明显的炎症反应。总的来说,这些发现强调了暴露于农药的卵巢中基因特异性DNA甲基化和炎症破坏。然而,缺乏ChIP检测限制了对DNMT招募的确认,这应该在未来的研究中得到解决。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


