Benzothiadiazole-thiophene based conjugated Polymers: Impact of conjugated chain length on electrochromic performance
IF 2.6
4区 工程技术
Q3 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this work, two donor-acceptor-donor (D-A-D) type electrochromic conjugated polymers were prepared upon electrochemical oxidation through their precursors employed thiophenes as the donor units and benzothiazole as the acceptor unit. The conjugated chain length effects of the polymers’ electrochemical and electrochromic properties were carefully examined. As the conjugated chain length increases, both precursors exhibited reduced the HOMO-LUMO energy gap and red-shifted UV–Vis absorption and fluorescence spectra. Both polymers displayed n-doping and p-doping process with excellent redox stability, PBT-4T retained 94.17 % of its original electroactivity, which is higher than that of PBT-2T (92.86 %). Meanwhile, both polymers exhibited reversible changes in UV–Vis absorption spectra under voltage drive of 0 V–1.8 V, accompanied by color changes from light pink to black-gray (PBT-2T) and from purple to dark brown (PBT-4T). PBT-2T showed highest optical contrast of 41 % at 750 nm and fastest response time of 0.2 s for the oxidation process at 528 nm because of porous structure, which undoubtedly facilitates the ingress and egress of ions. The varying the π-conjugated chain length from bithiophene to quaterthiophene strategy may provide a new research idea to achieve high-performance electrochromic conjugated polymers.
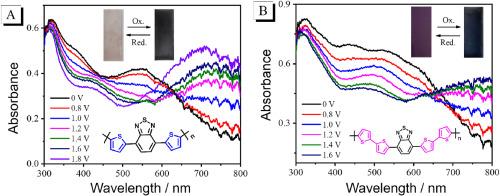
苯并噻唑-噻吩基共轭聚合物:共轭链长度对电致变色性能的影响
本文以噻吩为供体单元,苯并噻唑为受体单元,通过电化学氧化法制备了两种供体-受体-供体(D-A-D)型电致变色共轭聚合物。研究了共轭链长对聚合物电化学和电致变色性能的影响。随着共轭链长度的增加,两种前驱体的HOMO-LUMO能隙减小,紫外可见吸收光谱和荧光光谱红移。两种聚合物均表现为n掺杂和p掺杂过程,具有良好的氧化还原稳定性,PBT-4T保持了94.17%的原始电活性,高于PBT-2T的92.86%。同时,在0 V - 1.8 V电压驱动下,两种聚合物的UV-Vis吸收光谱均呈现可逆变化,颜色由浅粉色变为黑灰色(PBT-2T),由紫色变为深棕色(PBT-4T)。由于PBT-2T的多孔结构,其在750 nm处的光学对比度最高,达到41%,在528 nm处的氧化反应时间最快,为0.2 s,这无疑有利于离子的进出。从二噻吩到季噻吩改变π共轭链长度的策略可能为实现高性能电致变色共轭聚合物提供新的研究思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Electronics
工程技术-材料科学:综合
CiteScore
6.60
自引率
6.20%
发文量
238
审稿时长
44 days
期刊介绍:
Organic Electronics is a journal whose primary interdisciplinary focus is on materials and phenomena related to organic devices such as light emitting diodes, thin film transistors, photovoltaic cells, sensors, memories, etc.
Papers suitable for publication in this journal cover such topics as photoconductive and electronic properties of organic materials, thin film structures and characterization in the context of organic devices, charge and exciton transport, organic electronic and optoelectronic devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


