Tailored transethosomal systems for tadalafil transdermal delivery: Impact of Phosal and edge activators on skin permeation and cellular uptake
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
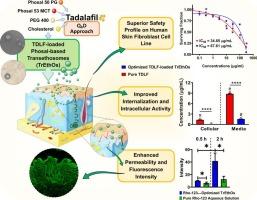
Tadalafil (TDLF), a Biopharmaceutics Classification System (BCS) Class II drug, exhibits poor aqueous solubility and extensive first-pass metabolism, which limits its therapeutic efficacy. We developed Phosal-based transethosomes (TrEthOs) to overcome these challenges, thereby enhancing transdermal delivery. A Box-Behnken design was employed to optimize the formulation by evaluating the effects of Phosal type, polyethylene glycol (PEG) 400 concentration, and cholesterol content. The optimized TrEthOs exhibited a mean vesicle size of 129.74 nm and an entrapment efficiency of 67.3%, ensuring efficient encapsulation of the drug. Ex vivo permeation studies demonstrated a cumulative TDLF permeation of 70.24% over 6 hours, with a steady-state flux of 19.49 × 10-4 mg/cm2·min. Confocal laser scanning microscopy confirmed deep skin penetration, while in vitro studies revealed significantly enhanced cellular uptake (P < 0.0001) and reduced cytotoxicity (IC₅₀ = 67.61 μg/mL) compared to pure TDLF (IC₅₀ = 34.85 μg/mL). The novel Phosal-based TrEthOs system presents a promising transdermal drug delivery strategy, potentially reducing dosing frequency and improving patient compliance. These outcomes emphasize the potential of advanced nanocarrier systems to optimize systemic bioavailability while minimizing adverse effects.
为他达拉非透皮递送量身定制的经体系统:磷和边缘激活剂对皮肤渗透和细胞摄取的影响
他达拉非(TDLF)是生物制药分类系统(BCS)第二类药物,其水溶性差,首过代谢广泛,限制了其治疗效果。为了克服这些挑战,我们开发了基于phosol的transsethosome (TrEthOs),从而增强了透皮递送。采用Box-Behnken设计,通过评价磷类型、聚乙二醇(PEG) 400浓度和胆固醇含量的影响来优化配方。优化后的TrEthOs平均囊泡大小为129.74 nm,包封效率为67.3%,确保了药物的高效包封。体外渗透研究表明,TDLF在6小时内的累计渗透率为70.24%,稳态通量为19.49 × 10-4 mg/cm2·min。共聚焦激光扫描显微镜证实皮肤深度穿透,而体外研究显示,与纯TDLF (IC₅₀= 34.85 μg/mL)相比,细胞吸收(P < 0.0001)显着增强,细胞毒性(IC₅₀= 67.61 μg/mL)降低。基于phosal的新型TrEthOs系统提出了一种很有前途的经皮给药策略,有可能减少给药频率并提高患者的依从性。这些结果强调了先进的纳米载体系统在优化系统生物利用度的同时最小化不良反应的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


