Workflow for evaluating enzyme immobilization and performance for continuous flow manufacturing
IF 4
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Enzymes have shown promise in various industries due to their functional specificity, catalytic efficiency, and environmental sustainability. These biological catalysts can be a pivotal component of manufacturing pipelines like continuous flow chemistry. For this, there exists a need to robustly immobilize enzymes on solid supports and assess the effects of the solid supports on catalytic performance and stability. Here, we use an industrially relevant model enzyme, C. ensiformis (Jack bean) urease, to demonstrate immobilization and assess performance in the context of continuous flow manufacturing. Various immobilization strategies were screened focusing on immobilization efficiency, protocol simplicity, and urease biocatalyst kinetics. Based on this, CDI-agarose and NHS-agarose resins were identified as the best-performing immobilization strategies for urease. CDI-agarose-urease and NHS-agarose-urease were then scaled up and applied to a large-scale continuous flow reactor to evaluate product yields, operational stability, and long-term stability. These experiments identified differences in stability and performance depending on the immobilization method tested. This highlights the importance of screening immobilization methods and subsequent enzyme performance for each candidate biocatalyst used in manufacturing to promote optimal performance and stability. As such, this work provides a framework for evaluating enzyme biocatalyst immobilization approaches to improve performance and enable transition into industrial processes.
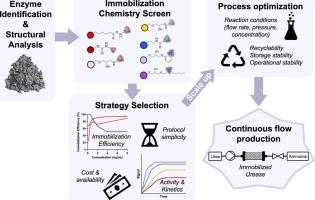
评估酶固定和连续流程生产性能的工作流程
酶由于其功能特异性、催化效率和环境可持续性,在各个行业都显示出前景。这些生物催化剂可以成为制造管道的关键组成部分,如连续流化学。为此,有必要将酶固定在固体载体上,并评估固体载体对催化性能和稳定性的影响。在这里,我们使用一种工业上相关的模型酶,C. ensiformis(杰克豆)脲酶,来演示固定和评估在连续流制造背景下的性能。对不同的固定化策略进行了筛选,重点是固定化效率、方案简单性和脲酶生物催化剂动力学。基于此,cdi -琼脂糖和nhs -琼脂糖树脂被确定为脲酶的最佳固定策略。然后将cdi -琼脂糖脲酶和nhs -琼脂糖脲酶放大并应用于大型连续流反应器,以评估产品收率、操作稳定性和长期稳定性。这些实验确定了稳定性和性能的差异取决于所测试的固定方法。这突出了筛选固定方法和随后用于制造的每种候选生物催化剂的酶性能的重要性,以促进最佳性能和稳定性。因此,这项工作为评估酶生物催化剂固定化方法提供了一个框架,以提高性能并使其能够过渡到工业过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Biotechnology
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
6.70
自引率
3.60%
发文量
50
审稿时长
38 days
期刊介绍:
Current Research in Biotechnology (CRBIOT) is a new primary research, gold open access journal from Elsevier. CRBIOT publishes original papers, reviews, and short communications (including viewpoints and perspectives) resulting from research in biotechnology and biotech-associated disciplines.
Current Research in Biotechnology is a peer-reviewed gold open access (OA) journal and upon acceptance all articles are permanently and freely available. It is a companion to the highly regarded review journal Current Opinion in Biotechnology (2018 CiteScore 8.450) and is part of the Current Opinion and Research (CO+RE) suite of journals. All CO+RE journals leverage the Current Opinion legacy-of editorial excellence, high-impact, and global reach-to ensure they are a widely read resource that is integral to scientists' workflow.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


