Co-combustion characteristics of ammonia and ethanol: A combined ReaxFF-MD and DFT study
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
Ammonia (NH3), as a "carbon-free" hydrogen energy carrier, is an excellent alternative to traditional fuels. It can improve combustion efficiency when co-burning with ethanol (C2H5OH). However, the research on the co-combustion characteristics of these two substances at the microscopic level is still incomplete. Therefore, the methods of ReaxFF molecular dynamics simulation and density functional theory are adopted to explore the combustion mechanisms of NH3 and C2H5OH. The calculation results show that C2H5OH first pyrolyzes thermally to generate free radicals such as ·OH and ·H, which promotes the oxidation of NH3 through a series of hydrogen abstraction reactions. Among them, the ·OH free radical obtains a kinetic advantage with its lowest energy barrier, thus playing a major role in promoting the oxidation of NH3. During the oxidation process, the amino group generates a variety of different intermediate products with the transfer of nitrogen elements, such as HNO, NH, and N2H4. The alkyl radicals ·CH3 and ·C2H5 mainly participate in the subsequent cyanidation reaction with ·NH2 and finally generate HCN. These findings provide mechanistic insights that support kinetic model development and nitrogen-species control strategies.
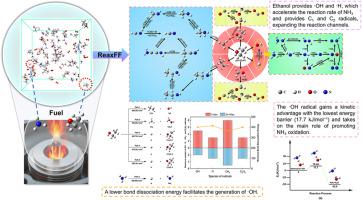
氨和乙醇共燃烧特性:ReaxFF-MD和DFT联合研究
氨(NH3)作为一种“无碳”氢能载体,是传统燃料的极好替代品。与乙醇(C2H5OH)共烧可提高燃烧效率。然而,在微观层面上对这两种物质共燃特性的研究尚不完整。因此,采用ReaxFF分子动力学模拟和密度泛函理论的方法,探讨NH3和C2H5OH的燃烧机理。计算结果表明,C2H5OH首先热热解生成·OH和·H等自由基,通过一系列吸氢反应促进NH3氧化。其中·OH自由基具有最低能垒的动力学优势,在促进NH3氧化中起主要作用。在氧化过程中,氨基随着氮元素的转移产生多种不同的中间产物,如HNO、NH、N2H4等。烷基自由基·CH3和·C2H5主要参与随后与·NH2的氰化反应,最终生成HCN。这些发现提供了支持动力学模型开发和氮物种控制策略的机制见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


