Boosting CO oxidation performance via reaction-induced single-atom active site: DFT and microkinetic investigation of a unified CO oxidation mechanism on Gd-doped ceria-supported copper catalysts
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
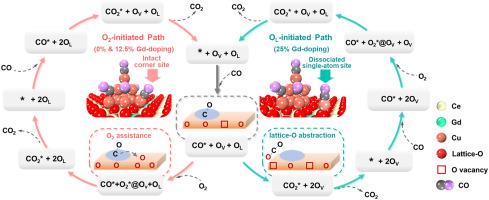
Single-atom (SA) catalysts have emerged as a pivotal area drawing extensive research interest due to their high catalytic activities. However, SA catalysts are often plagued by the aggregation and deactivation of SA sites under reaction conditions. This study focuses on CO oxidation over Gd-doped ceria-supported Cu catalysts and aims to provide a new strategy to stabilize the SA site, in which a Cu SA site is “prestored” in a relatively stable Cu cluster and can be dynamically activated under reaction conditions. Three typical Cu10/CeO2 catalyst models were built with different Gd-doping contents, which are pristine Cu10/CeO2, Cu10/Gd0.125Ce0.875O2, and Cu10/Gd0.25Ce0.75O2, respectively. We performed density functional theory (DFT) calculations on the Cu10/Gd-CeO2 system to investigate the adsorption of CO and O2 molecules, the formation of surface oxygen vacancy (OV) and dynamic Cu SA site, and potential energy surfaces of CO oxidation process. Ab initio thermodynamic analysis suggests that the saturation adsorption of CO on Cu10 and high Gd-doping in CeO2 lead to a spontaneously formed single Cu−CO site and an OV defect on ceria surface. The CO oxidation process is identified as a two-paths-coupled catalytic cycle, in which Path I is activated by the terminal O atom of adsorbed O2 at surface OV site while Path II initiates with the lattice O atom of CeO2 surface. The microkinetic modeling demonstrates that the dominant pathway is Path I for the undoped and low-doping cases, and Path II for the high-doping case which exhibits a novel mechanism for CO oxidation and the highest reaction activity due to the participation of the dynamic SA site.
通过反应诱导的单原子活性位点提高CO氧化性能:gd掺杂铈负载铜催化剂CO氧化统一机理的DFT和微动力学研究
单原子催化剂由于具有较高的催化活性而成为一个重要的研究领域。然而,在反应条件下,SA催化剂经常受到SA位点聚集和失活的困扰。本研究的重点是在gd掺杂的铈负载Cu催化剂上进行CO氧化,旨在提供一种稳定SA位点的新策略,其中Cu SA位点被“预先储存”在相对稳定的Cu簇中,并可以在反应条件下动态激活。建立了三种不同gd掺杂量的Cu10/CeO2催化剂模型,分别为原始Cu10/CeO2、Cu10/Gd0.125Ce0.875O2和Cu10/Gd0.25Ce0.75O2。通过密度泛函理论(DFT)计算Cu10/Gd-CeO2体系对CO和O2分子的吸附、表面氧空位(OV)和动态Cu SA位点的形成以及CO氧化过程的势能面。从头算热力学分析表明,CO在Cu10上的饱和吸附和CeO2中高含量的gd掺杂导致了氧化铈表面自发形成单个Cu−CO位和OV缺陷。CO氧化过程是一个双路径耦合的催化循环,其中路径I是由表面OV位点吸附O2的末端O原子激活的,而路径II是由CeO2表面晶格O原子启动的。微动力学模型表明,未掺杂和低掺杂情况下的主要途径是路径I,高掺杂情况下的主要途径是路径II,由于动态SA位点的参与,路径II表现出新的CO氧化机制和最高的反应活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


