Vacant ZnAl-layered double hydroxides photocatalytic CO2 to CH4: A DFT study
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photocatalytic reduction of carbon dioxide (CO2PR) into hydrocarbons is one of the most promising technologies to address global energy demand and environmental issues, and vacancy engineering can significantly enhance the activity of photocatalysts in CO2PR. Herein, the structure, charge properties of vacant ZnAl-LDHs (hydrogen vacancy: VH, hydroxyl vacancy: VOH, zinc vacancy: VZn) and the thermodynamic mechanism of CO2PR over these LDHs are investigated by using density functional theory. The calculation results indicate that the introduction of vacancies can promote the adsorption and activation of reactant CO2. Compared to perfect ZnAl-LDH, introducing vacancies can reduce the Gibbs free energy barriers for CO2PR to CO and CH4. The analysis of the Gibbs free energy barriers of potential-determining step (ΔGPDS) and HER suggests that VOH-ZnAl-LDH is the best CO2PR catalyst. By comparing the adsorption of reactants and intermediate species at different positions on the catalyst, it is found that species adsorption at vacancies is more conducive to the occurrence of catalytic reactions. Regulating the OH concentrations on the surface of LDHs also affects the catalytic activity of the catalyst, and when the concentration is 2/9, it has the lowest Gibbs free energy (0.258 eV) barrier for CO2PR to CH4. This work provides theoretical guidance for understanding the photocatalytic performance of LDHs materials containing vacancies for CO2PR, and provides useful directions for designing and preparing efficient CO2PR catalysts.
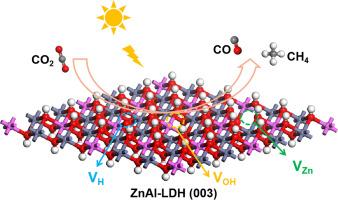
空锌层双氢氧化物光催化CO2制CH4的DFT研究
光催化还原二氧化碳(CO2PR)为碳氢化合物是解决全球能源需求和环境问题最有前途的技术之一,而空位工程可以显著提高光催化剂在CO2PR中的活性。本文利用密度泛函理论研究了空ZnAl-LDHs(氢空位:VH,羟基空位:VOH,锌空位:VZn)的结构、电荷性质以及CO2PR在这些LDHs上的热力学机理。计算结果表明,空位的引入可以促进CO2的吸附和活化。与完美的ZnAl-LDH相比,引入空位可以降低CO2PR对CO和CH4的吉布斯自由能垒。势决定步Gibbs自由能垒(ΔGPDS)和HER分析表明,VOH-ZnAl-LDH是最佳的CO2PR催化剂。通过比较反应物和中间物在催化剂上不同位置的吸附情况,发现空位处的物质吸附更有利于催化反应的发生。调节LDHs表面OH浓度也会影响催化剂的催化活性,当浓度为2/9时,CO2PR转化为CH4的吉布斯自由能最低(0.258 eV)。本研究为了解含CO2PR空位的LDHs材料的光催化性能提供了理论指导,并为设计和制备高效的CO2PR催化剂提供了有益的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


