Process development of zeolite-Y-catalyst from Kulende MetaKaolin: Experimental data modelling, process optimization, computer-aided scale-up techno-economics and zeolite-Y-catalyst application for biodiesel production
Q1 Environmental Science
引用次数: 0
Abstract
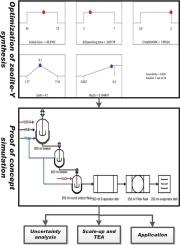
Most existing studies on zeolite-Y synthesis remain at the laboratory-scale, limiting process design and industrial application. This study optimized key variables: acid concentration, quench time, and NaOH:KMK for the synthesis of zeolite-Y from the Kulende Metakaolin. The optimal zeolite-Y synthesis condition was simulated, scaled-up and its feasibility study was performed through Techno economic analysis. The synthesized zeolite-Y was tested in the transesterification process. Optimal conditions (65.97 % acid, 3.89 min quenching, and 1.99 NaOH:KMK) was used for zeolite-Y synthesis. The lab-optimized process was simulated and scaled up to 4000 kg/batch, achieving 104 batches/year, 0.65 kg/min production rate, and 5.8 million kJ total energy consumed. Techno-economic analysis confirmed feasibility, with a 15-year investment yielding a NPV of $9.59 million, 27.23 % IRR, and 27 % ROI. The synthesized zeolite-Y was successfully tested for biodiesel production, with both products respectively characterized. This study demonstrates that zeolite-Y from KMK is scalable, economically viable, and suitable for industrial biodiesel production.
库伦德MetaKaolin沸石- y催化剂工艺开发:实验数据建模、工艺优化、计算机辅助放大技术经济及沸石- y催化剂在生物柴油生产中的应用
现有的大多数沸石- y合成研究仍停留在实验室规模,限制了工艺设计和工业应用。本研究优化了酸浓度、淬火时间、NaOH:KMK等关键参数对库伦德偏高岭土合成y型沸石的影响。对最佳沸石- y合成条件进行了模拟、放大,并通过技术经济分析进行了可行性研究。对合成的y沸石进行了酯交换反应。最佳工艺条件为:酸度为65.97%,淬火时间为3.89 min, NaOH:KMK为1.99。对实验室优化工艺进行了模拟,并扩大到4000 kg/批次,实现了104批次/年、0.65 kg/min的生产速率、580万kJ的总能耗。技术经济分析证实了该项目的可行性,15年投资的净现值为959万美元,内部收益率为27.23%,投资回报率为27%。合成的沸石- y成功用于生物柴油的生产,并对两种产品分别进行了表征。该研究表明,KMK的沸石y具有可扩展性,经济可行性,适合工业生物柴油生产。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioresource Technology Reports
Environmental Science-Environmental Engineering
CiteScore
7.20
自引率
0.00%
发文量
390
审稿时长
28 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


