Inhibition of Gremlin1 reduces osteoclast activation and alleviates inflammation-induced bone loss by disrupting the NF-κB signaling pathway
IF 2.3
Q1 DENTISTRY, ORAL SURGERY & MEDICINE
引用次数: 0
Abstract
Objectives
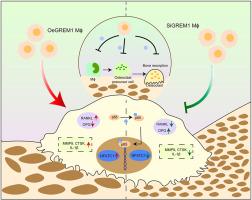
Gremlin1 plays a crucial role in the development of various inflammatory diseases that cause bone loss, including periodontitis and apical periodontitis (AP). The impact of Gremlin1 on osteoclast activation and its mechanisms in the progression of inflammation-induced bone loss remains unclear. This study aimed to investigate the role of Gremlin1 in the activation of osteoclasts within the inflamed peri-apical region and its possible mechanism.
Methods
A local Gremlin1 knock-down AP animal model was established. Micro-computed tomography and hematoxylin and eosin staining were used to assess the volume of bone defects and the extent of inflammatory infiltration. Tartrate-resistant acid phosphatase staining was used to quantify osteoclast numbers and immunohistochemical staining was used to assess the expression of osteoclast differentiation-related proteins in inflamed peri-apical tissues. Subsequently, THP-1 cells with differential expression of Gremlin1 were stimulated to develop into osteoclasts and the activation of osteoclasts and variables associated with osteoclastogenesis were re-evaluated. The activation level of the NF-κB signaling pathway was examined using in vivo and in vitro models.
Results
Local administration of adeno-associated virus-silenced Gremlin1 to peri-apical tissues significantly inhibited alveolar bone loss. The number of osteoclasts and the expression of genes associated with osteoclastogenesis were markedly decreased. The in vivo and in vitro experimental findings were consistent and revealed that the silencing of Gremlin1 effectively suppresses activation of the NF-κB signaling pathway. Conversely, over-expression of Gremlin1 may facilitate activation of this pathway.
Conclusion
Gremlin1 regulates osteoclasts differentiation through the NF-κB signaling pathway, thereby attenuating the development of inflammation-induced bone loss.
抑制Gremlin1可通过破坏NF-κB信号通路降低破骨细胞活化,减轻炎症诱导的骨质流失
目的gremlin1在导致骨质流失的各种炎症性疾病(包括牙周炎和根尖牙周炎(AP))的发展中起关键作用。Gremlin1对破骨细胞活化的影响及其在炎症性骨质流失进展中的机制尚不清楚。本研究旨在探讨Gremlin1在炎症根尖周围区域破骨细胞活化中的作用及其可能的机制。方法建立局部Gremlin1敲除型AP动物模型。显微计算机断层扫描和苏木精、伊红染色评估骨缺损的体积和炎症浸润的程度。采用抗酒石酸酸性磷酸酶染色定量破骨细胞数量,免疫组化染色评估炎症根尖周围组织中破骨细胞分化相关蛋白的表达。随后,刺激差异表达Gremlin1的THP-1细胞发育成破骨细胞,并重新评估破骨细胞的活化和与破骨细胞发生相关的变量。采用体内和体外模型检测NF-κB信号通路的激活水平。结果在根尖周围组织局部注射腺相关病毒沉默的Gremlin1可显著抑制牙槽骨丢失。破骨细胞数量和破骨细胞生成相关基因表达明显减少。体内和体外实验结果一致,表明沉默Gremlin1可有效抑制NF-κB信号通路的激活。相反,过度表达Gremlin1可能促进该途径的激活。结论gremlin1通过NF-κB信号通路调控破骨细胞分化,从而减轻炎症性骨质流失的发生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Oral Biosciences
DENTISTRY, ORAL SURGERY & MEDICINE-
CiteScore
4.40
自引率
12.50%
发文量
57
审稿时长
37 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


